Abstract
Families in which both parents are heterozygotes for the same autosomal dominant neoplasia syndrome are extremely unusual. Recently, we had the unique opportunity to evaluate three symptomatic siblings from the union between two unrelated individuals affected by multiple endocrine neoplasia type 1 (MEN1). When the three siblings and their parents and relatives were genotyped for 12 markers tightly linked to the MEN1 locus, at 11q13, two of the siblings were found to be homozygotes, and one a heterozygote, for MEN1. With regard to the MEN1 syndrome, no phenotypic differences were observed between the two homozygotes and the heterozygotes. However, the two homozygotes showed unexplained infertility, which was not the case for any of the heterozygotes. Thus, MEN1 appears to be a disease with complete dominance, and the presence of two MEN1 alleles with mutations of the type that occur constitutionally may be insufficient for tumor development.
Full text
PDF

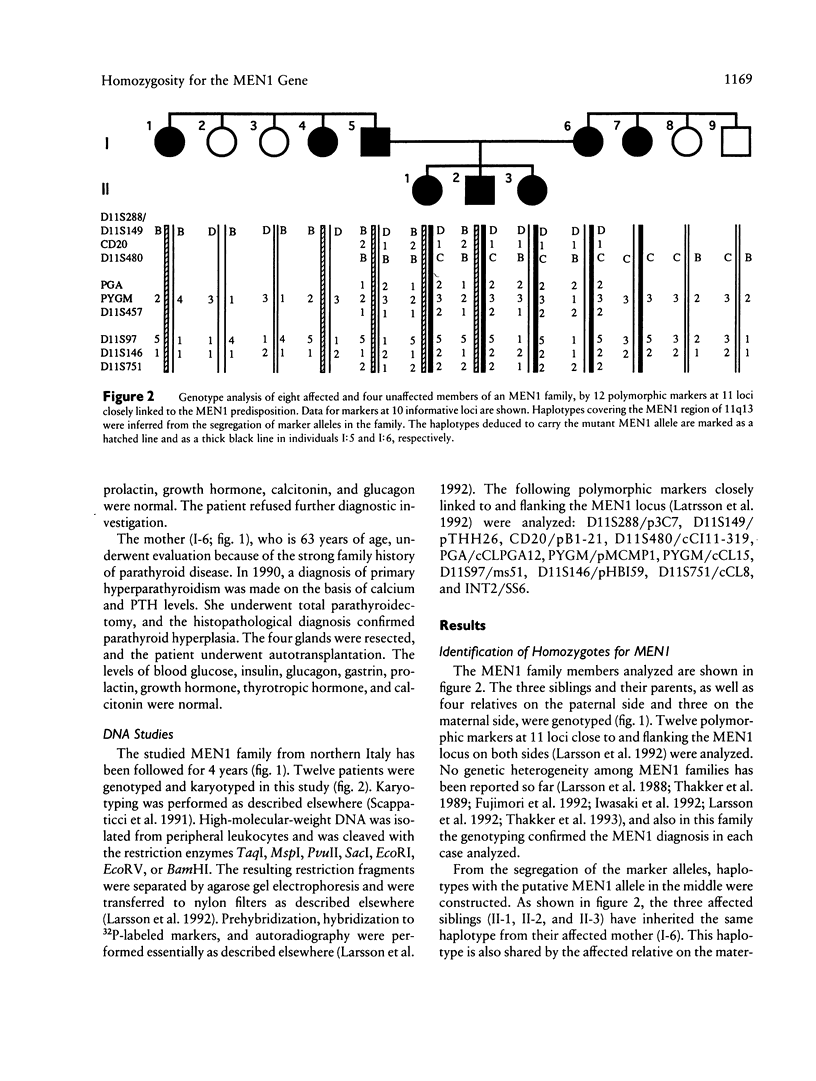



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandi M. L., Marx S. J., Aurbach G. D., Fitzpatrick L. A. Familial multiple endocrine neoplasia type I: a new look at pathophysiology. Endocr Rev. 1987 Nov;8(4):391–405. doi: 10.1210/edrv-8-4-391. [DOI] [PubMed] [Google Scholar]
- Byström C., Larsson C., Blomberg C., Sandelin K., Falkmer U., Skogseid B., Oberg K., Werner S., Nordenskjöld M. Localization of the MEN1 gene to a small region within chromosome 11q13 by deletion mapping in tumors. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1968–1972. doi: 10.1073/pnas.87.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Maandag E. R., van Roon M., van der Lugt N. M., van der Valk M., Hooper M. L., Berns A., te Riele H. Requirement for a functional Rb-1 gene in murine development. Nature. 1992 Sep 24;359(6393):328–330. doi: 10.1038/359328a0. [DOI] [PubMed] [Google Scholar]
- Donehower L. A., Harvey M., Slagle B. L., McArthur M. J., Montgomery C. A., Jr, Butel J. S., Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992 Mar 19;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Farndon J. R., Leight G. S., Dilley W. G., Baylin S. B., Smallridge R. C., Harrison T. S., Wells S. A., Jr Familial medullary thyroid carcinoma without associated endocrinopathies: a distinct clinical entity. Br J Surg. 1986 Apr;73(4):278–281. doi: 10.1002/bjs.1800730411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E., De Marco L., Gejman P. V., Norton J. A., Bale A. E., Aurbach G. D., Spiegel A. M., Marx S. J. Allelic loss from chromosome 11 in parathyroid tumors. Cancer Res. 1992 Dec 15;52(24):6804–6809. [PubMed] [Google Scholar]
- Friedman E., Sakaguchi K., Bale A. E., Falchetti A., Streeten E., Zimering M. B., Weinstein L. S., McBride W. O., Nakamura Y., Brandi M. L. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med. 1989 Jul 27;321(4):213–218. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fujimori M., Wells S. A., Jr, Nakamura Y. Fine-scale mapping of the gene responsible for multiple endocrine neoplasia type 1 (MEN 1). Am J Hum Genet. 1992 Feb;50(2):399–403. [PMC free article] [PubMed] [Google Scholar]
- Hall J. G. Genomic imprinting and its clinical implications. N Engl J Med. 1992 Mar 19;326(12):827–829. doi: 10.1056/NEJM199203193261210. [DOI] [PubMed] [Google Scholar]
- Henry I., Bonaiti-Pellié C., Chehensse V., Beldjord C., Schwartz C., Utermann G., Junien C. Uniparental paternal disomy in a genetic cancer-predisposing syndrome. Nature. 1991 Jun 20;351(6328):665–667. doi: 10.1038/351665a0. [DOI] [PubMed] [Google Scholar]
- Heutink P., van der Mey A. G., Sandkuijl L. A., van Gils A. P., Bardoel A., Breedveld G. J., van Vliet M., van Ommen G. J., Cornelisse C. J., Oostra B. A. A gene subject to genomic imprinting and responsible for hereditary paragangliomas maps to chromosome 11q23-qter. Hum Mol Genet. 1992 Apr;1(1):7–10. doi: 10.1093/hmg/1.1.7. [DOI] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Iwasaki H., Stewart P. W., Dilley W. G., Holt M. S., Steinbrueck T. D., Wells S. A., Jr, Donis-Keller H. A minisatellite and a microsatellite polymorphism within 1.5 kb at the human muscle glycogen phosphorylase (PYGM) locus can be amplified by PCR and have combined informativeness of PIC 0.95. Genomics. 1992 May;13(1):7–15. doi: 10.1016/0888-7543(92)90194-w. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Julier C., Nakamura Y., Lathrop M., O'Connell P., Leppert M., Litt M., Mohandas T., Lalouel J. M., White R. A detailed genetic map of the long arm of chromosome 11. Genomics. 1990 Jul;7(3):335–345. doi: 10.1016/0888-7543(90)90167-s. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Shepherd J., Nakamura Y., Blomberg C., Weber G., Werelius B., Hayward N., Teh B., Tokino T., Seizinger B. Predictive testing for multiple endocrine neoplasia type 1 using DNA polymorphisms. J Clin Invest. 1992 Apr;89(4):1344–1349. doi: 10.1172/JCI115720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., van Beersum S. E., Cremers C. W., van Baars F. M., Ropers H. H. Analysis of a second family with hereditary non-chromaffin paragangliomas locates the underlying gene at the proximal region of chromosome 11q. Hum Genet. 1993 May;91(4):357–361. doi: 10.1007/BF00217356. [DOI] [PubMed] [Google Scholar]
- Nordström S., Thorburn W. Dominantly inherited macular degeneration (Best's disease) in a homozygous father with 11 children. Clin Genet. 1980 Sep;18(3):211–216. doi: 10.1111/j.1399-0004.1980.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Scappaticci S., Maraschio P., del Ciotto N., Fossati G. S., Zonta A., Fraccaro M. Chromosome abnormalities in lymphocytes and fibroblasts of subjects with multiple endocrine neoplasia type 1. Cancer Genet Cytogenet. 1991 Mar;52(1):85–92. doi: 10.1016/0165-4608(91)90057-2. [DOI] [PubMed] [Google Scholar]
- Skogseid B., Eriksson B., Lundqvist G., Lörelius L. E., Rastad J., Wide L., Akerström G., Oberg K. Multiple endocrine neoplasia type 1: a 10-year prospective screening study in four kindreds. J Clin Endocrinol Metab. 1991 Aug;73(2):281–287. doi: 10.1210/jcem-73-2-281. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Bouloux P., Wooding C., Chotai K., Broad P. M., Spurr N. K., Besser G. M., O'Riordan J. L. Association of parathyroid tumors in multiple endocrine neoplasia type 1 with loss of alleles on chromosome 11. N Engl J Med. 1989 Jul 27;321(4):218–224. doi: 10.1056/NEJM198907273210403. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Wooding C., Pang J. T., Farren B., Harding B., Anderson D. C., Besser G. M., Bouloux P., Brenton D. P., Buchanan K. D. Linkage analysis of 7 polymorphic markers at chromosome 11p11.2-11q13 in 27 multiple endocrine neoplasia type 1 families. Ann Hum Genet. 1993 Jan;57(Pt 1):17–25. doi: 10.1111/j.1469-1809.1993.tb00883.x. [DOI] [PubMed] [Google Scholar]
- Wexler N. S., Young A. B., Tanzi R. E., Travers H., Starosta-Rubinstein S., Penney J. B., Snodgrass S. R., Shoulson I., Gomez F., Ramos Arroyo M. A. Homozygotes for Huntington's disease. Nature. 1987 Mar 12;326(6109):194–197. doi: 10.1038/326194a0. [DOI] [PubMed] [Google Scholar]


