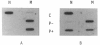Abstract
The lysosomal removal of the sulfate moiety from sulfatide requires the action of two proteins, arylsulfatase A and sphingolipid activator protein-1 (SAP-1). Recently, patients have been identified who have a variant form of metachromatic leukodystrophy which is characterized by mutations in the gene coding for SAP-1, which is also called "prosaposin." All of the mutations characterized in these patients result in (a) deficient mature SAP-1, as determined by immunoblotting after SDS-PAGE of tissue and cell extracts, and (b) decreased ability of cultured skin fibroblasts to metabolize endocytosed [14C]-sulfatide. We now report the insertion of the full-length prosaposin cDNA into the Moloney murine leukemia virus-derived retroviral vector, pLJ, and the infection of cultured skin fibroblasts from a newly diagnosed and molecularly characterized patient with SAP-1 deficiency. The cultured cells infected with the prosaposin cDNA construct now show both production of normal levels of mature SAP-1 and completely normal metabolism of endocytosed [14C]-sulfatide. These studies demonstrate that the virally transferred prosaposin cDNA is processed normally and is localized within lysosomes, where it is needed for interaction between sulfatide and arylsulfatase A. In addition, normal as well as mutant sequences can now be found by allele-specific oligonucleotide hybridization of PCR-amplified genomic DNA by using exonic sequences as primers.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christomanou H., Aignesberger A., Linke R. P. Immunochemical characterization of two activator proteins stimulating enzymic sphingomyelin degradation in vitro. Absence of one of them in a human Gaucher disease variant. Biol Chem Hoppe Seyler. 1986 Sep;367(9):879–890. doi: 10.1515/bchm3.1986.367.2.879. [DOI] [PubMed] [Google Scholar]
- Conzelmann E., Sandhoff K. AB variant of infantile GM2 gangliosidosis: deficiency of a factor necessary for stimulation of hexosaminidase A-catalyzed degradation of ganglioside GM2 and glycolipid GA2. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3979–3983. doi: 10.1073/pnas.75.8.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewji N. N., Wenger D. A., O'Brien J. S. Nucleotide sequence of cloned cDNA for human sphingolipid activator protein 1 precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8652–8656. doi: 10.1073/pnas.84.23.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewji N., Wenger D., Fujibayashi S., Donoviel M., Esch F., Hill F., O'Brien J. S. Molecular cloning of the sphingolipid activator protein-1 (SAP-1), the sulfatide sulfatase activator. Biochem Biophys Res Commun. 1986 Jan 29;134(2):989–994. doi: 10.1016/s0006-291x(86)80518-6. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Inui K., Wenger D. A. Activator protein-deficient metachromatic leukodystrophy: diagnosis in leukocytes using immunologic methods. J Pediatr. 1984 May;104(5):739–742. doi: 10.1016/s0022-3476(84)80957-9. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Wenger D. A. Biosynthesis of the sulfatide/GM1 activator protein (SAP-1) in control and mutant cultured skin fibroblasts. Biochim Biophys Acta. 1986 Feb 28;875(3):554–562. doi: 10.1016/0005-2760(86)90077-9. [DOI] [PubMed] [Google Scholar]
- Fürst W., Machleidt W., Sandhoff K. The precursor of sulfatide activator protein is processed to three different proteins. Biol Chem Hoppe Seyler. 1988 May;369(5):317–328. doi: 10.1515/bchm3.1988.369.1.317. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hahn A. F., Gordon B. A., Hinton G. G., Gilbert J. J. A variant form of metachromatic leukodystrophy without arylsulfatase deficiency. Ann Neurol. 1982 Jul;12(1):33–36. doi: 10.1002/ana.410120106. [DOI] [PubMed] [Google Scholar]
- Harzer K., Paton B. C., Poulos A., Kustermann-Kuhn B., Roggendorf W., Grisar T., Popp M. Sphingolipid activator protein deficiency in a 16-week-old atypical Gaucher disease patient and his fetal sibling: biochemical signs of combined sphingolipidoses. Eur J Pediatr. 1989 Oct;149(1):31–39. doi: 10.1007/BF02024331. [DOI] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtschmidt H., Sandhoff K., Kwon H. Y., Harzer K., Nakano T., Suzuki K. Sulfatide activator protein. Alternative splicing that generates three mRNAs and a newly found mutation responsible for a clinical disease. J Biol Chem. 1991 Apr 25;266(12):7556–7560. [PubMed] [Google Scholar]
- Inui K., Emmett M., Wenger D. A. Immunological evidence for deficiency in an activator protein for sulfatide sulfatase in a variant form of metachromatic leukodystrophy. Proc Natl Acad Sci U S A. 1983 May;80(10):3074–3077. doi: 10.1073/pnas.80.10.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui K., Kao F. T., Fujibayashi S., Jones C., Morse H. G., Law M. L., Wenger D. A. The gene coding for a sphingolipid activator protein, SAP-1, is on human chromosome 10. Hum Genet. 1985;69(3):197–200. doi: 10.1007/BF00293023. [DOI] [PubMed] [Google Scholar]
- Inui K., Wenger D. A. Biochemical, immunological, and structural studies on a sphingolipid activator protein (SAP-1). Arch Biochem Biophys. 1984 Sep;233(2):556–564. doi: 10.1016/0003-9861(84)90479-x. [DOI] [PubMed] [Google Scholar]
- Korman A. J., Frantz J. D., Strominger J. L., Mulligan R. C. Expression of human class II major histocompatibility complex antigens using retrovirus vectors. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2150–2154. doi: 10.1073/pnas.84.8.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz K. A., Carson G. S., Morimoto S., Kishimoto Y., Fluharty A. L., O'Brien J. S. Characterization of a mutation in a family with saposin B deficiency: a glycosylation site defect. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2541–2544. doi: 10.1073/pnas.87.7.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudoh T., Wenger D. A. Diagnosis of metachromatic leukodystrophy, Krabbe disease, and Farber disease after uptake of fatty acid-labeled cerebroside sulfate into cultured skin fibroblasts. J Clin Invest. 1982 Jul;70(1):89–97. doi: 10.1172/JCI110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Eine Cerebrosidsulfatase aus Schweineniere. Hoppe Seylers Z Physiol Chem. 1964;339(1):260–276. [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Kishimoto Y., O'Brien J. S. Saposin D: a sphingomyelinase activator. Biochem Biophys Res Commun. 1988 Oct 14;156(1):403–410. doi: 10.1016/s0006-291x(88)80855-6. [DOI] [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Yamamoto Y., Kretz K. A., O'Brien J. S., Kishimoto Y. Saposin A: second cerebrosidase activator protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3389–3393. doi: 10.1073/pnas.86.9.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Sandhoff K., Stümper J., Christomanou H., Suzuki K. Structure of full-length cDNA coding for sulfatide activator, a Co-beta-glucosidase and two other homologous proteins: two alternate forms of the sulfatide activator. J Biochem. 1989 Feb;105(2):152–154. doi: 10.1093/oxfordjournals.jbchem.a122629. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kretz K. A., Dewji N., Wenger D. A., Esch F., Fluharty A. L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988 Aug 26;241(4869):1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- Rafi M. A., Zhang X. L., DeGala G., Wenger D. A. Detection of a point mutation in sphingolipid activator protein-1 mRNA in patients with a variant form of metachromatic leukodystrophy. Biochem Biophys Res Commun. 1990 Jan 30;166(2):1017–1023. doi: 10.1016/0006-291x(90)90912-7. [DOI] [PubMed] [Google Scholar]
- Rorman E. G., Grabowski G. A. Molecular cloning of a human co-beta-glucosidase cDNA: evidence that four sphingolipid hydrolase activator proteins are encoded by single genes in humans and rats. Genomics. 1989 Oct;5(3):486–492. doi: 10.1016/0888-7543(89)90014-1. [DOI] [PubMed] [Google Scholar]
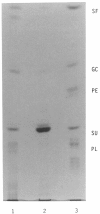
- Stevens R. L., Fluharty A. L., Kihara H., Kaback M. M., Shapiro L. J., Marsh B., Sandhoff K., Fischer G. Cerebroside sulfatase activator deficiency induced metachromatic leukodystrophy. Am J Hum Genet. 1981 Nov;33(6):900–906. [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., DeGala G., Williams C., Taylor H. A., Stevenson R. E., Pruitt J. R., Miller J., Garen P. D., Balentine J. D. Clinical, pathological, and biochemical studies on an infantile case of sulfatide/GM1 activator protein deficiency. Am J Med Genet. 1989 Jun;33(2):255–265. doi: 10.1002/ajmg.1320330223. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Sattler M., Roth S. A protein activator of galactosylceramide beta-galactosidase. Biochim Biophys Acta. 1982 Sep 14;712(3):639–649. doi: 10.1016/0005-2760(82)90293-4. [DOI] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Zhang X. L., Rafi M. A., DeGala G., Wenger D. A. Insertion in the mRNA of a metachromatic leukodystrophy patient with sphingolipid activator protein-1 deficiency. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1426–1430. doi: 10.1073/pnas.87.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]