Abstract
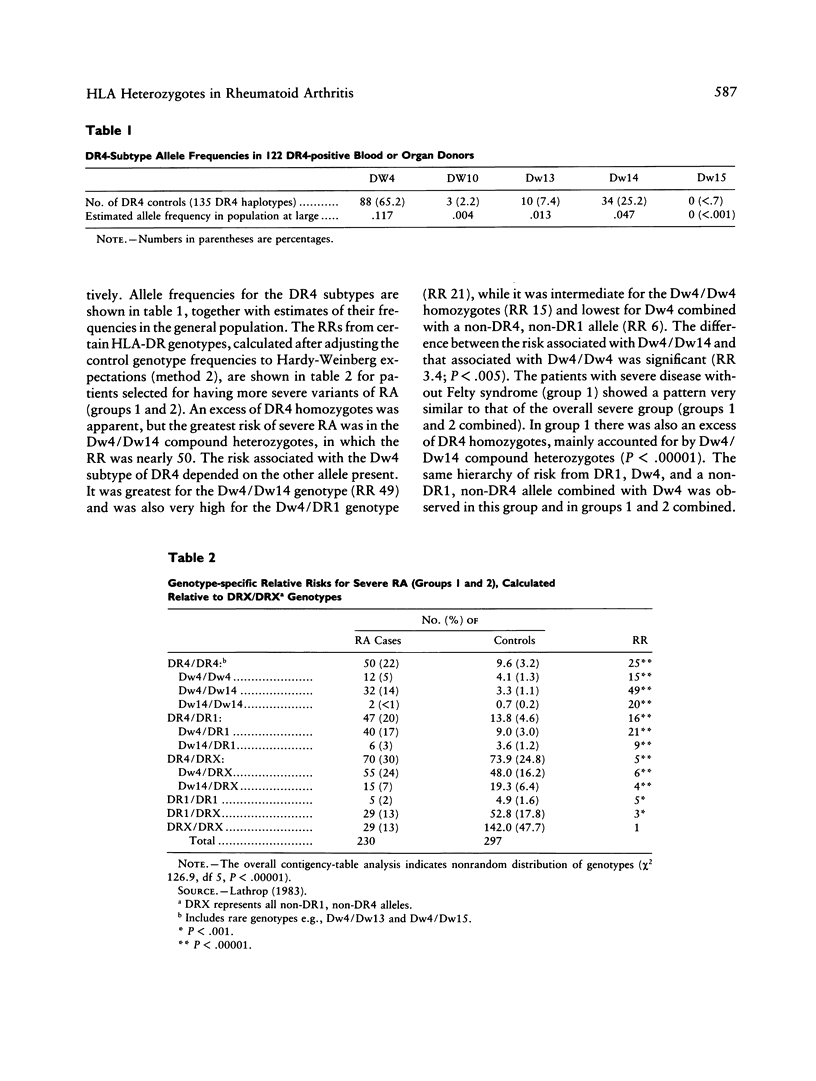
We have investigated the role of HLA-DR genotypes in 184 patients with severe rheumatoid arthritis (RA) and in 46 patients with Felty syndrome, to establish the relative contribution of the RA-associated subtypes of DR4 (Dw4, Dw14, and Dw15). There was an excess of DR4 homozygotes, particularly Dw4/Dw14 compound heterozygotes (relative risk [RR] 49). The risk associated with Dw4 depended on the other allele present--Dw4/DR1 (RR 21), Dw4/Dw4 (RR 15), and Dw4/DRX (RR 6). There was a significant risk from Dw4/Dw14 compared with Dw4/Dw4, both in those with severe RA (RR 2.9; P less than .02) and in those with Felty syndrome (RR 4.2; P less than .02). In contrast, in a further 63 known DR4 homozygotes with RA, not selected for severe disease, the excess of Dw4/Dw14 was much less striking (RR 1.4; not significant), suggesting that this genotype may be particularly associated with more severe disease. We also found four cases with the rare Dw4/Dw15 genotype (expected less than or equal to 0.5; P less than or equal to .02). Since the Dw4, Dw14, Dw15, and DR1 molecules have similar antigen-binding sites and since combinations of these alleles particularly predispose to severe RA, we suggest that synergistic mechanisms are involved. These could include an effect on T-cell repertoire selection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Brown M. G., Driscoll J., Monaco J. J. Structural and serological similarity of MHC-linked LMP and proteasome (multicatalytic proteinase) complexes. Nature. 1991 Sep 26;353(6342):355–357. doi: 10.1038/353355a0. [DOI] [PubMed] [Google Scholar]
- Campion G., Maddison P. J., Goulding N., James I., Ahern M. J., Watt I., Sansom D. The Felty syndrome: a case-matched study of clinical manifestations and outcome, serologic features, and immunogenetic associations. Medicine (Baltimore) 1990 Mar;69(2):69–80. [PubMed] [Google Scholar]
- Davis M. M. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- Gao X. J., Olsen N. J., Pincus T., Stastny P. HLA-DR alleles with naturally occurring amino acid substitutions and risk for development of rheumatoid arthritis. Arthritis Rheum. 1990 Jul;33(7):939–946. doi: 10.1002/art.1780330704. [DOI] [PubMed] [Google Scholar]
- Glynne R., Powis S. H., Beck S., Kelly A., Kerr L. A., Trowsdale J. A proteasome-related gene between the two ABC transporter loci in the class II region of the human MHC. Nature. 1991 Sep 26;353(6342):357–360. doi: 10.1038/353357a0. [DOI] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Jaraquemada D., Ollier W., Awad J., Young A., Silman A., Roitt I. M., Corbett M., Hay F., Cosh J. A., Maini R. N. HLA and rheumatoid arthritis: a combined analysis of 440 British patients. Ann Rheum Dis. 1986 Aug;45(8):627–636. doi: 10.1136/ard.45.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M. A., Yamashita T. S., Reynolds T. L., Wolfe F., Khan M. K. HLA-DR4 genotype frequency and gender effect in familial rheumatoid arthritis. Tissue Antigens. 1988 May;31(5):254–258. doi: 10.1111/j.1399-0039.1988.tb02092.x. [DOI] [PubMed] [Google Scholar]
- Lanchbury J. S., Hall M. A., Welsh K. I., Panayi G. S. Sequence analysis of HLA-DR4B1 subtypes: additional first domain variability is detected by oligonucleotide hybridization and nucleotide sequencing. Hum Immunol. 1990 Feb;27(2):136–144. doi: 10.1016/0198-8859(90)90110-b. [DOI] [PubMed] [Google Scholar]
- Lanchbury J. S., Jaeger E. E., Sansom D. M., Hall M. A., Wordsworth P., Stedeford J., Bell J. I., Panayi G. S. Strong primary selection for the Dw4 subtype of DR4 accounts for the HLA-DQw7 association with Felty's syndrome. Hum Immunol. 1991 Sep;32(1):56–64. doi: 10.1016/0198-8859(91)90117-r. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M. Estimating genotype relative risks. Tissue Antigens. 1983 Aug;22(2):160–166. doi: 10.1111/j.1399-0039.1983.tb01183.x. [DOI] [PubMed] [Google Scholar]
- Maeda H., Juji T., Mitsui H., Sonozaki H., Okitsu K. HLA DR4 and rheumatoid arthritis in Japanese people. Ann Rheum Dis. 1981 Jun;40(3):299–302. doi: 10.1136/ard.40.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott M., Molloy M., Cashin P., McMahon M., Spencer S., Jennings S., Sneyd A., Greally J., Silman A., Ollier W. A multicase family study of rheumatoid arthritis in south west Ireland. Dis Markers. 1986 Jun;4(1-2):103–111. [PubMed] [Google Scholar]
- Nepom B. S., Nepom G. T., Mickelson E., Schaller J. G., Antonelli P., Hansen J. A. Specific HLA-DR4-associated histocompatibility molecules characterize patients with seropositive juvenile rheumatoid arthritis. J Clin Invest. 1984 Jul;74(1):287–291. doi: 10.1172/JCI111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Seyfried C. E., Holbeck S. L., Wilske K. R., Nepom B. S. Identification of HLA-Dw14 genes in DR4+ rheumatoid arthritis. Lancet. 1986 Nov 1;2(8514):1002–1005. doi: 10.1016/s0140-6736(86)92614-0. [DOI] [PubMed] [Google Scholar]
- Nicklas J. A., Noreen H. J., Segall M., Bach F. H. Southern analysis of DNA polymorphism among Dw subtypes of DR4. Hum Immunol. 1985 Jun;13(2):95–103. doi: 10.1016/0198-8859(85)90016-3. [DOI] [PubMed] [Google Scholar]
- Ollier W., Silman A., Gosnell N., Currey H., Awad J., Doyle P., McCloskey D., Alonso A., Hossain M. A., Festenstein H. HLA and rheumatoid arthritis: an analysis of multicase families. Dis Markers. 1986 Jun;4(1-2):85–98. [PubMed] [Google Scholar]
- Payami H., Thomson G., Khan M. A., Grennan D. M., Sanders P., Dyer P., Dostal C. Genetics of rheumatoid arthritis. Tissue Antigens. 1986 Feb;27(2):57–63. doi: 10.1111/j.1399-0039.1986.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Pile K. D., Tikly M., Bell J. I., Wordsworth B. P. HLA-DR antigens and rheumatoid arthritis in black South Africans: a study of ethnic groups. Tissue Antigens. 1992 Mar;39(3):138–140. doi: 10.1111/j.1399-0039.1992.tb01924.x. [DOI] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Rigby A. S., Silman A. J., Voelm L., Gregory J. C., Ollier W. E., Khan M. A., Nepom G. T., Thomson G. Investigating the HLA component in rheumatoid arthritis: an additive (dominant) mode of inheritance is rejected, a recessive mode is preferred. Genet Epidemiol. 1991;8(3):153–175. doi: 10.1002/gepi.1370080303. [DOI] [PubMed] [Google Scholar]
- Sheehy M. J., Scharf S. J., Rowe J. R., Neme de Gimenez M. H., Meske L. M., Erlich H. A., Nepom B. S. A diabetes-susceptible HLA haplotype is best defined by a combination of HLA-DR and -DQ alleles. J Clin Invest. 1989 Mar;83(3):830–835. doi: 10.1172/JCI113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G., Robinson W. P., Kuhner M. K., Joe S., MacDonald M. J., Gottschall J. L., Barbosa J., Rich S. S., Bertrams J., Baur M. P. Genetic heterogeneity, modes of inheritance, and risk estimates for a joint study of Caucasians with insulin-dependent diabetes mellitus. Am J Hum Genet. 1988 Dec;43(6):799–816. [PMC free article] [PubMed] [Google Scholar]
- Willkens R. F., Nepom G. T., Marks C. R., Nettles J. W., Nepom B. S. Association of HLA-Dw16 with rheumatoid arthritis in Yakima Indians. Further evidence for the "shared epitope" hypothesis. Arthritis Rheum. 1991 Jan;34(1):43–47. doi: 10.1002/art.1780340107. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Allsopp C. E., Young R. P., Bell J. I. HLA-DR typing using DNA amplification by the polymerase chain reaction and sequential hybridization to sequence-specific oligonucleotide probes. Immunogenetics. 1990;32(6):413–418. doi: 10.1007/BF00241635. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P. HLA class II antigens in susceptibility to rheumatoid arthritis. Br J Rheumatol. 1991 Apr;30(2):151–152. doi: 10.1093/rheumatology/30.2.151. [DOI] [PubMed] [Google Scholar]
- Wordsworth B. P., Lanchbury J. S., Sakkas L. I., Welsh K. I., Panayi G. S., Bell J. I. HLA-DR4 subtype frequencies in rheumatoid arthritis indicate that DRB1 is the major susceptibility locus within the HLA class II region. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10049–10053. doi: 10.1073/pnas.86.24.10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordsworth B. P., Stedeford J., Rosenberg W. M., Bell J. I. Limited heterogeneity of the HLA class II contribution to susceptibility to rheumatoid arthritis is suggested by positive associations with HLA-DR4, DR1 and DRw10. Br J Rheumatol. 1991 Jun;30(3):178–180. doi: 10.1093/rheumatology/30.3.178. [DOI] [PubMed] [Google Scholar]


