Abstract
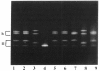
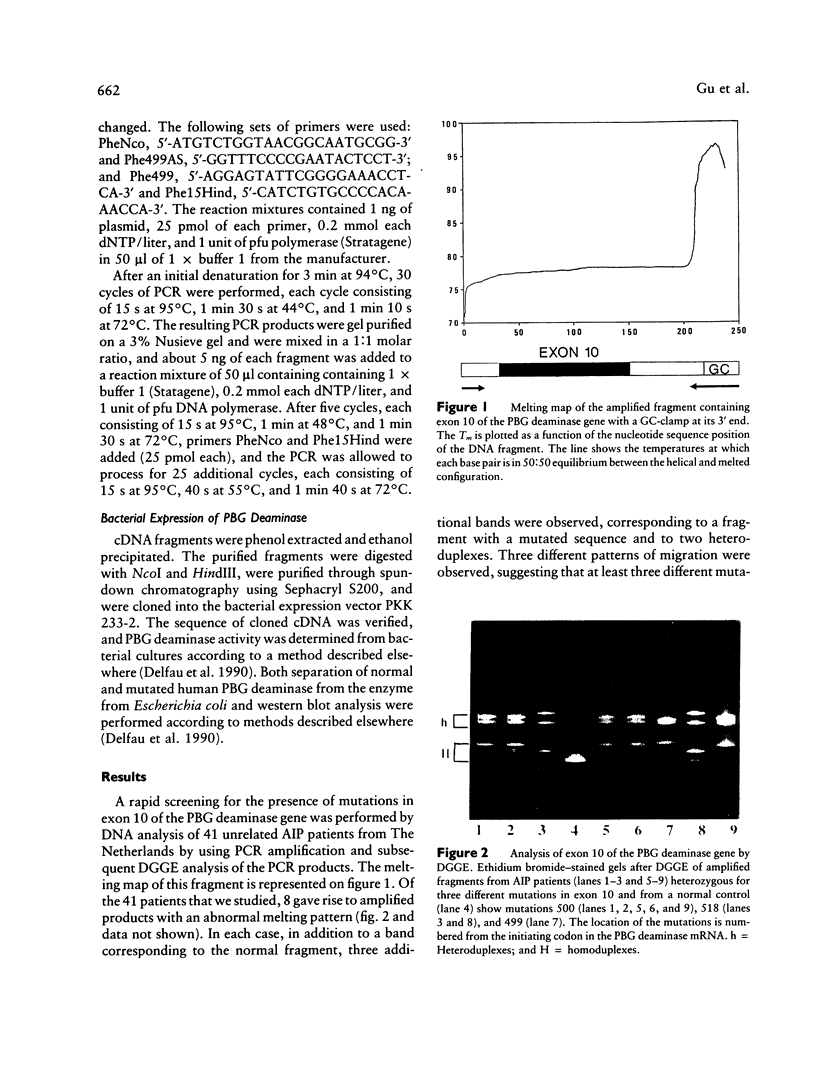
Acute intermittent porphyria (AIP) is an autosomal dominant disease characterized by a partial deficiency of porphobilinogen (PBG) deaminase. Different subtypes of the disease have been defined, and more than 10 different mutations have been described. We focused our study on exon 10, since we previously found that three different mutations were located in this exon and that two of them seemed to be relatively common. We used denaturing gradient gel electrophoresis (DGGE) after in vitro amplification to detect all possible mutations in exon 10 in 41 unrelated AIP patients. In about one-fourth of these patients we could distinguish three abnormal migration patterns, indicating the presence of various mutations. Additional sequencing demonstrated the presence of three different single-base substitutions. Two of these mutations had already been described. A third one consisted of a C-to-T transition located at position 499 of the PBG deaminase mRNA and resulted in an Arg-to-Trp substitution. All three mutations were found in patients with cross-reacting immunological material (CRIM)-positive forms of AIP. The high frequency of these mutations make DGGE analysis of exon 10 a useful approach allowing the direct direction of the DNA abnormality in most of the families with the CRIM-positive subtype of AIP.
Full text
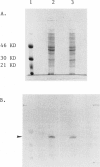
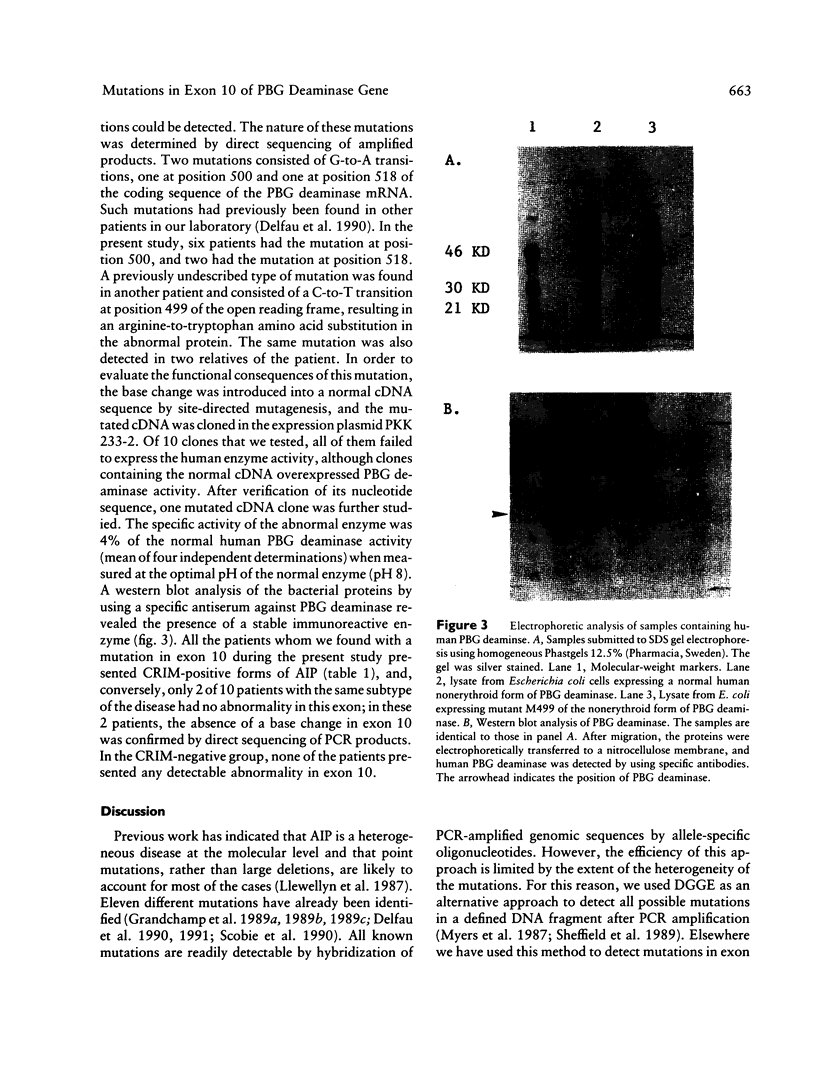
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaumont C., Porcher C., Picat C., Nordmann Y., Grandchamp B. The mouse porphobilinogen deaminase gene. Structural organization, sequence, and transcriptional analysis. J Biol Chem. 1989 Sep 5;264(25):14829–14834. [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. The role of arginyl residues in porphyrin binding to ferrochelatase. J Biol Chem. 1986 Jun 15;261(17):7902–7905. [PubMed] [Google Scholar]
- Delfau M. H., Picat C., De Rooij F., Voortman G., Deybach J. C., Nordmann Y., Grandchamp B. Molecular heterogeneity of acute intermittent porphyria: identification of four additional mutations resulting in the CRIM-negative subtype of the disease. Am J Hum Genet. 1991 Aug;49(2):421–428. [PMC free article] [PubMed] [Google Scholar]
- Delfau M. H., Picat C., de Rooij F. W., Hamer K., Bogard M., Wilson J. H., Deybach J. C., Nordmann Y., Grandchamp B. Two different point G to A mutations in exon 10 of the porphobilinogen deaminase gene are responsible for acute intermittent porphyria. J Clin Invest. 1990 Nov;86(5):1511–1516. doi: 10.1172/JCI114869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnick R. J., Ostasiewicz L. T., Tishler P. A., Mustajoki P. Acute intermittent porphyria: characterization of a novel mutation in the structural gene for porphobilinogen deaminase. Demonstration of noncatalytic enzyme intermediates stabilized by bound substrate. J Clin Invest. 1985 Aug;76(2):865–874. doi: 10.1172/JCI112044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Kauppinen R., Mignotte V., Peltonen L., Mustajoki P., Roméo P. H., Goossens M., Nordmann Y. Molecular analysis of acute intermittent porphyria in a Finnish family with normal erythrocyte porphobilinogen deaminase. Eur J Clin Invest. 1989 Oct;19(5):415–418. doi: 10.1111/j.1365-2362.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., Mignotte V., Wilson J. H., Te Velde K., Sandkuyl L., Roméo P. H., Goossens M., Nordmann Y. Tissue-specific splicing mutation in acute intermittent porphyria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):661–664. doi: 10.1073/pnas.86.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandchamp B., Picat C., de Rooij F., Beaumont C., Wilson P., Deybach J. C., Nordmann Y. A point mutation G----A in exon 12 of the porphobilinogen deaminase gene results in exon skipping and is responsible for acute intermittent porphyria. Nucleic Acids Res. 1989 Aug 25;17(16):6637–6649. doi: 10.1093/nar/17.16.6637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman T., Ståhl S., Hornes E., Uhlén M. Direct solid phase sequencing of genomic and plasmid DNA using magnetic beads as solid support. Nucleic Acids Res. 1989 Jul 11;17(13):4937–4946. doi: 10.1093/nar/17.13.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Anvret M., Lindsten J., Lannfelt L., Gellerfors P., Wetterberg L., Floderus Y., Thunell S. DNA polymorphisms within the porphobilinogen deaminase gene in two Swedish families with acute intermittent porphyria. Hum Genet. 1988 Aug;79(4):379–381. doi: 10.1007/BF00282182. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Llewellyn D. H., Elder G. H., Kalsheker N. A., Marsh O. W., Harrison P. R., Grandchamp B., Picat C., Nordmann Y., Romeo P. H., Goossens M. DNA polymorphism of human porphobilinogen deaminase gene in acute intermittent porphyria. Lancet. 1987 Sep 26;2(8561):706–708. doi: 10.1016/s0140-6736(87)91073-7. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Scobie G. A., Llewellyn D. H., Urquhart A. J., Smyth S. J., Kalsheker N. A., Harrison P. R., Elder G. H. Acute intermittent porphyria caused by a C----T mutation that produces a stop codon in the porphobilinogen deaminase gene. Hum Genet. 1990 Oct;85(6):631–634. doi: 10.1007/BF00193588. [DOI] [PubMed] [Google Scholar]
- Sharif A. L., Smith A. G., Abell C. Isolation and characterisation of a cDNA clone for a chlorophyll synthesis enzyme from Euglena gracilis. The chloroplast enzyme hydroxymethylbilane synthase (porphobilinogen deaminase) is synthesised with a very long transit peptide in Euglena. Eur J Biochem. 1989 Sep 15;184(2):353–359. doi: 10.1111/j.1432-1033.1989.tb15026.x. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubnicer A. C., Picat C., Grandchamp B. Rat porphobilinogen deaminase cDNA: nucleotide sequence of the erythropoietic form. Nucleic Acids Res. 1988 Apr 11;16(7):3102–3102. doi: 10.1093/nar/16.7.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. D., Jordan P. M. Nucleotide sequence of the hemC locus encoding porphobilinogen deaminase of Escherichia coli K12. Nucleic Acids Res. 1986 Aug 11;14(15):6215–6226. doi: 10.1093/nar/14.15.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]