Abstract
Protoporphyria is a hereditary disorder characterized by a marked decrease in the activity of ferrochelatase, the terminal enzyme in the heme biosynthetic pathway. We have prepared specific polyvalent antibodies against bovine ferrochelatase in rabbits. The specificity of the antibody preparation against ferrochelatase was demonstrated by western blot analysis and immunoprecipitation of ferrochelatase activity. The antibody also cross-reacted weakly with ferrochelatase from human mitochondria. To quantify immunoreactive ferrochelatase in tissue samples, a kinetic-based enzyme-linked immunosorbent assay (k-ELISA) was developed. Ferrochelatase activity and the level of immunoreactive protein were measured in hepatic mitochondria isolated from six normal and nine protoporphyric (homozygous) cattle. Ferrochelatase activity was less than 10% of normal in mitochondria from protoporphyric animals; the amount of immunoreactive material was equivalent to that from normal animals. Similar studies were performed with samples from three normal and two protoporphyric (heterozygous) humans. Ferrochelatase activity was decreased in protoporphyric samples (about 17% of normal, but there was no concomitant decrease in immunoreactive material. These data demonstrate that a normal amount of ferrochelatase protein is present and suggest that bovine and human protoporphyria result from point mutations in the gene encoding ferrochelatase.
Full text
PDF


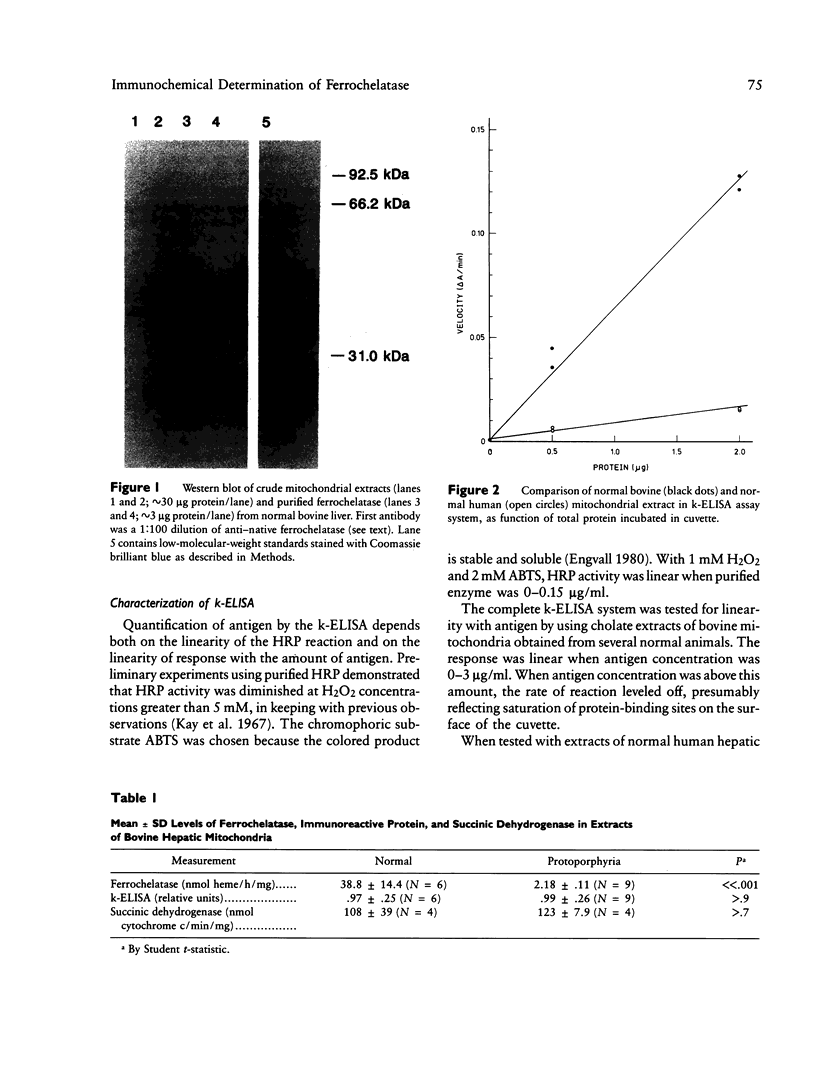
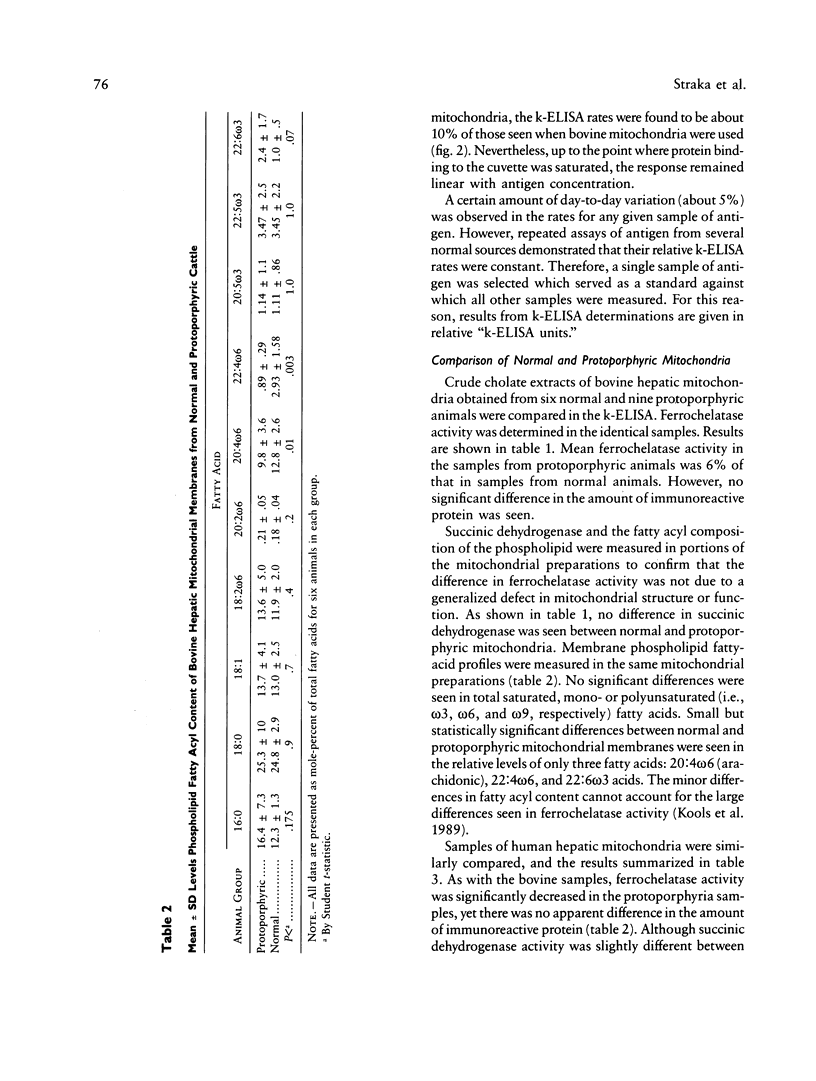
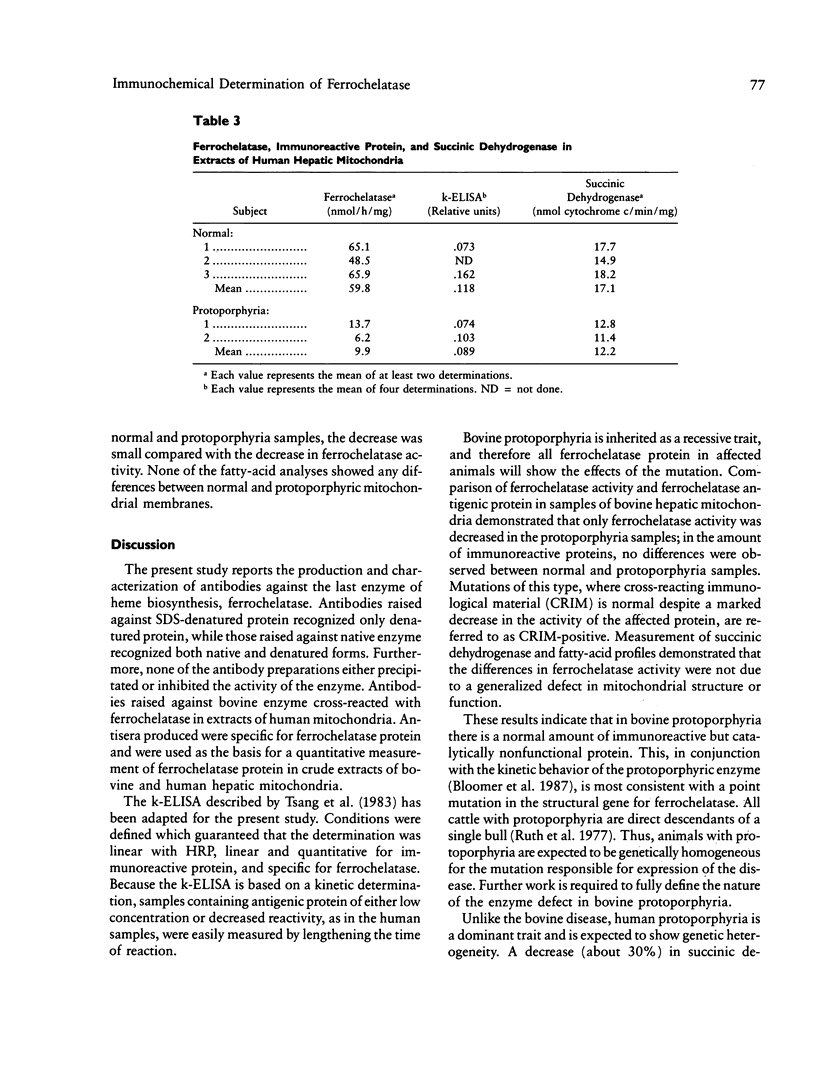

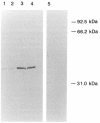
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloomer J. R., Hill H. D., Morton K. O., Anderson-Burnham L. A., Straka J. G. The enzyme defect in bovine protoporphyria. Studies with purified ferrochelatase. J Biol Chem. 1987 Jan 15;262(2):667–671. [PubMed] [Google Scholar]
- Bloomer J. R., Morton K. O., Reuter R. J., Ruth G. R. Bovine protoporphyria: documentation of autosomal recessive inheritance and comparison with the human disease through measurement of heme synthase activity. Am J Hum Genet. 1982 Mar;34(2):322–330. [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Dailey H. A., Fleming J. E. Bovine ferrochelatase. Kinetic analysis of inhibition by N-methylprotoporphyrin, manganese, and heme. J Biol Chem. 1983 Oct 10;258(19):11453–11459. [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Hancock K., Tsang V. C. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1983 Aug;133(1):157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- Harbin B. M., Dailey H. A. Orientation of ferrochelatase in bovine liver mitochondria. Biochemistry. 1985 Jan 15;24(2):366–370. doi: 10.1021/bi00323a019. [DOI] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Straka J. G. Protein determination using bicinchoninic acid in the presence of sulfhydryl reagents. Anal Biochem. 1988 Apr;170(1):203–208. doi: 10.1016/0003-2697(88)90109-1. [DOI] [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- Jones M. S., Jones O. T. The structural organization of haem synthesis in rat liver mitochondria. Biochem J. 1969 Jul;113(3):507–514. doi: 10.1042/bj1130507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- Kools A. M., Straka J. G., Hill H. D., Whitmer D. I., Holman R. T., Bloomer J. R. Modulation of hepatic ferrochelatase activity by dietary manipulation of mitochondrial phospholipid fatty acyl groups. Hepatology. 1989 Apr;9(4):557–561. doi: 10.1002/hep.1840090409. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morton K. O., Schneider F., Weimer M. K., Straka J. G., Bloomer J. R. Hepatic and bile porphyrins in patients with protoporphyria and liver failure. Gastroenterology. 1988 Jun;94(6):1488–1492. doi: 10.1016/0016-5085(88)90690-7. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Vitols K. S., Labbe R. F., Newton N. A. Studies on ferrochelatase. The effects of thiols and other factors on the determination of activity. Biochem J. 1967 Aug;104(2):321–327. doi: 10.1042/bj1040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTH G. R., Schwartz S., Stephenson B. Bovine protoporphyria: the first nonhuman model of this hereditary photosensitizing disease. Science. 1977 Oct 14;198(4313):199–201. doi: 10.1126/science.905823. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Tokunaga R. Purification and substrate specificity of bovine liver-ferrochelatase. Eur J Biochem. 1982 Oct;127(3):443–447. doi: 10.1111/j.1432-1033.1982.tb06892.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Wilson B. C., Peralta J. M. Quantitative, single-tube, kinetic-dependent enzyme-linked immunosorbent assay (k-ELISA). Methods Enzymol. 1983;92:391–403. doi: 10.1016/0076-6879(83)92033-5. [DOI] [PubMed] [Google Scholar]