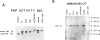Abstract
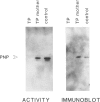
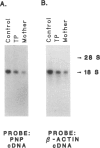
Purine nucleoside phosphorylase (PNP) deficiency is an inherited autosomal recessive disorder resulting in severe combined immunodeficiency. The purpose of this study was to determine the molecular defects responsible for PNP deficiency in one such patient. The patient's PNP cDNA was amplified by PCR and sequenced. Point mutations leading to amino acid substitutions were found in both alleles. One point mutation led to a Ser-to-Gly substitution at amino acid 51 and was common to both alleles. In addition, an Asp-to-Gly substitution at amino acid 128 and an Arg-to-Pro substitution at amino acid 234 were found in the maternal and paternal alleles, respectively. In order to prove that these mutations were responsible for the disease state, each of the three mutations was constructed separately by site-directed mutagenesis of the normal PNP cDNA, and each was transiently expressed in COS cells. Lysates from cells transfected with the allele carrying the substitution at amino acid 51 retained both function and immunoreactivity. Lysates from cells transfected with PNP alleles carrying a substitution at either amino acid 128 or amino acid 234 contained immunoreactive material but had no detectable human PNP activity. In summary, molecular analysis of this patient identified point mutations within the PNP gene which are responsible for the enzyme deficiency.
Full text
PDF



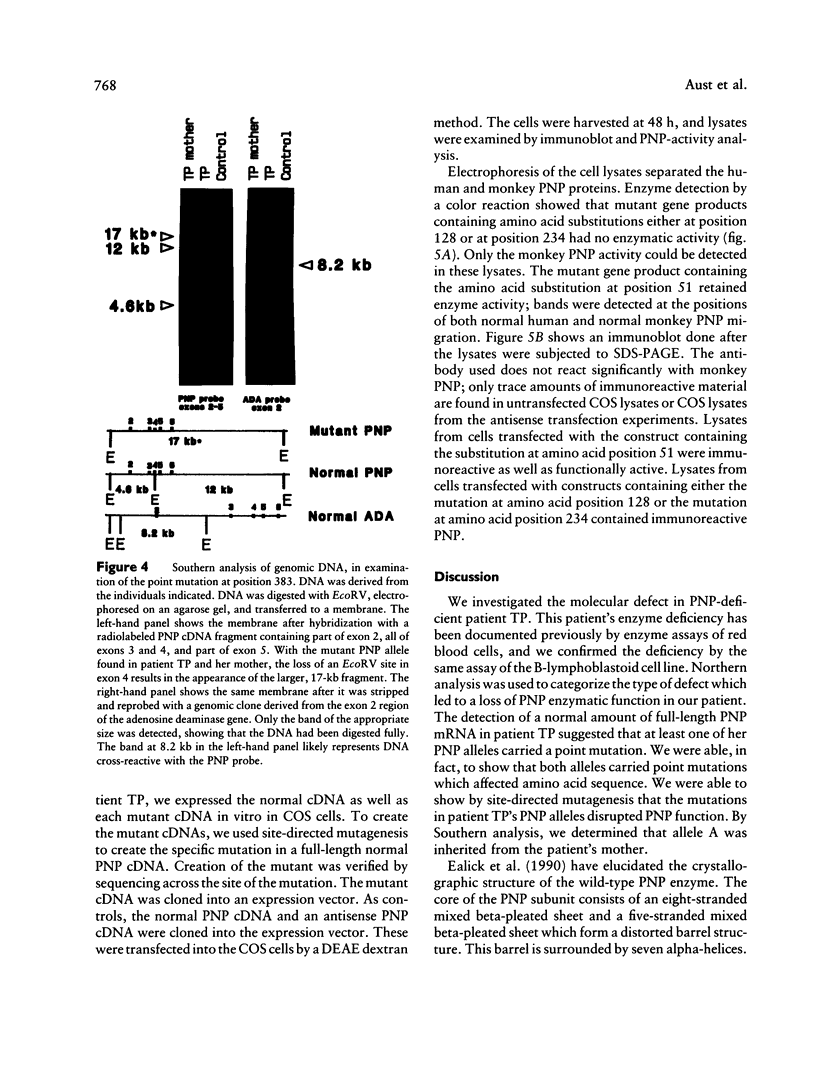
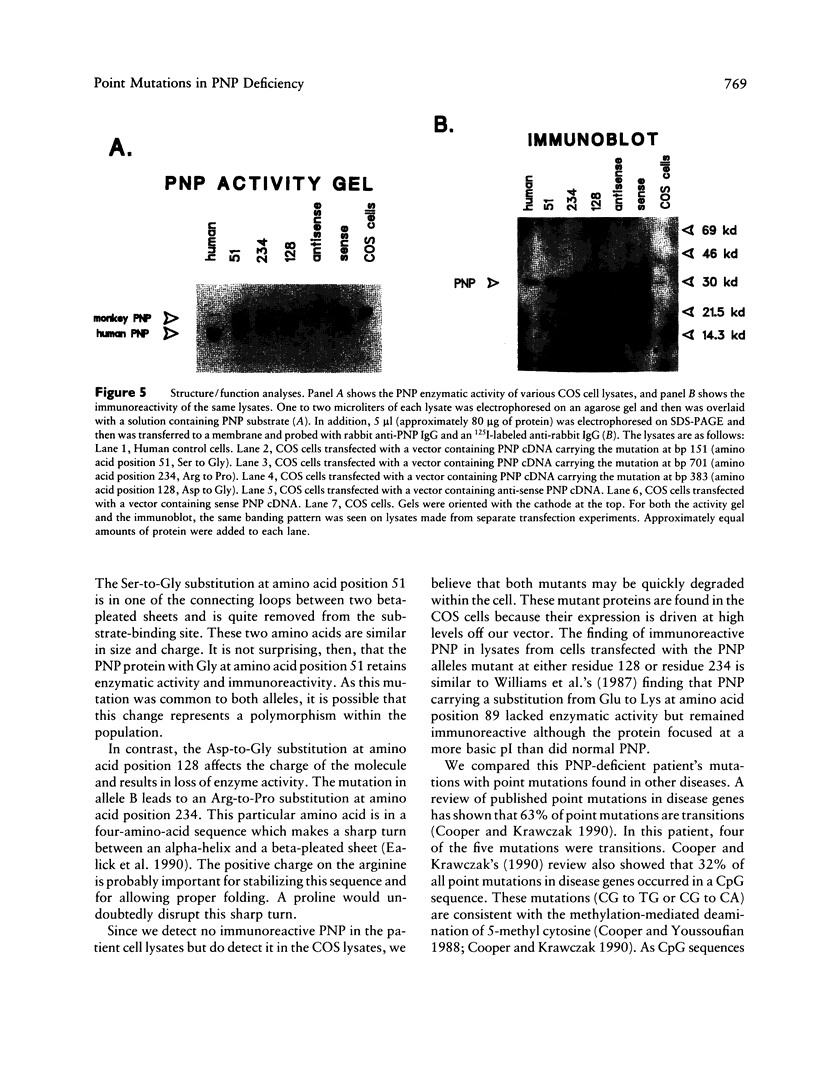



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E., Jr Purine nucleoside phosphorylase from human erythrocytes. IV. Crystallization and some properties. J Biol Chem. 1969 Feb 25;244(4):644–647. [PubMed] [Google Scholar]
- Ammann A. J., Wara D. W., Allen T. Immunotherapy and immunopathologic studies in a patient with nucleoside phosphorylase deficiency. Clin Immunol Immunopathol. 1978 Jul;10(3):262–269. doi: 10.1016/0090-1229(78)90180-0. [DOI] [PubMed] [Google Scholar]
- Anderson M. A., Gusella J. F. Use of cyclosporin A in establishing Epstein-Barr virus-transformed human lymphoblastoid cell lines. In Vitro. 1984 Nov;20(11):856–858. doi: 10.1007/BF02619631. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio Corrales F., Madero L., Zabay J. M., Ludeña M. C., Gómez de la Concha E., Lozano C., Sainz T. Déficit de purina nucleósido fosforilasa. Presentación de dos casos. An Esp Pediatr. 1983 Mar;18(3):248–253. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carapella-de Luca E., Aiuti F., Lucarelli P., Bruni L., Baroni C. D., Imperato C., Roos D., Astaldi A. A patient with nucleoside phosphorylase deficiency, selective t-cell deficiency, and autoimmune hemolytic anemia. J Pediatr. 1978 Dec;93(6):1000–1003. doi: 10.1016/s0022-3476(78)81237-2. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Krawczak M. The mutational spectrum of single base-pair substitutions causing human genetic disease: patterns and predictions. Hum Genet. 1990 Jun;85(1):55–74. doi: 10.1007/BF00276326. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Didsbury J., Weber R. F., Bokoch G. M., Evans T., Snyderman R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J Biol Chem. 1989 Oct 5;264(28):16378–16382. [PubMed] [Google Scholar]
- Ealick S. E., Rule S. A., Carter D. C., Greenhough T. J., Babu Y. S., Cook W. J., Habash J., Helliwell J. R., Stoeckler J. D., Parks R. E., Jr Three-dimensional structure of human erythrocytic purine nucleoside phosphorylase at 3.2 A resolution. J Biol Chem. 1990 Jan 25;265(3):1812–1820. doi: 10.2210/pdb2pnp/pdb. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Andres C. M., Gelfand E. W., Biggar D. Purine nucleoside phosphorylase deficiency: altered kinetic properties of a mutant enzyme. Science. 1977 Sep 9;197(4308):1084–1086. doi: 10.1126/science.407651. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Greenberg M. L., Chaffee S., Hershfield M. S. Basis for resistance to 3-deazaaristeromycin, an inhibitor of S-adenosylhomocysteine hydrolase, in human B-lymphoblasts. J Biol Chem. 1989 Jan 15;264(2):795–803. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Zannis V. I., Clift S. M., Ammann A. J., Staal G. E., Martin D. W., Jr Characterization of mutant subunits of human purine nucleoside phosphorylase. J Biol Chem. 1978 Dec 25;253(24):8916–8924. [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Jonsson J. J., Williams S. R., McIvor R. S. Sequence and functional characterization of the human purine nucleoside phosphorylase promoter. Nucleic Acids Res. 1991 Sep 25;19(18):5015–5020. doi: 10.1093/nar/19.18.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki T., Hinuma Y. Characteristics of cell lines derived from human leukocytes transformed by different strains of Epstein-Barr virus. Int J Cancer. 1975 Feb 15;15(2):203–210. doi: 10.1002/ijc.2910150205. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Schiff R. I., Buckley R. H. Adenosine deaminase and purine nucleoside phosphorylase deficiencies: evaluation of therapeutic interventions in eight patients. J Clin Immunol. 1987 Sep;7(5):389–399. doi: 10.1007/BF00917017. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Wiginton D. A., States J. C., Ward F. E., Bigner S. H., Buckley R. H., Kaufman R. E., Hutton J. J. Identification of a deletion in the adenosine deaminase gene in a child with severe combined immunodeficiency. J Immunol. 1987 May 15;138(10):3203–3206. [PubMed] [Google Scholar]
- Markert M. L. Purine nucleoside phosphorylase deficiency. Immunodefic Rev. 1991;3(1):45–81. [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- McGinniss M. H., Wasniowska K., Zopf D. A., Straus S. E., Reichert C. M. An erythrocyte Pr auto-antibody with sialoglycoprotein specificity in a patient with purine nucleoside phosphorylase deficiency. Transfusion. 1985 Mar-Apr;25(2):131–136. doi: 10.1046/j.1537-2995.1985.25285169204.x. [DOI] [PubMed] [Google Scholar]
- McRoberts J. A., Martin D. W., Jr Submolecular characterization of a mutant human purine-nucleoside phosphorylase. J Biol Chem. 1980 Jun 25;255(12):5605–5615. [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Chen S. H., Giblett E. R., Biggar W. D., Ammann A. A., Scott C. R. Purine nucleoside phosphorylase deficiency. Evidence for molecular heterogeneity in two families with enzyme-deficient members. J Clin Invest. 1977 Sep;60(3):741–746. doi: 10.1172/JCI108826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciuti F., Ruddle F. H. Assignment of nucleoside phosphorylase to D-14 and localization of X-linked loci in man by somatic cell genetics. Nat New Biol. 1973 Feb 7;241(110):180–182. doi: 10.1038/newbio241180a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoop J. W., Zegers B. J., Hendrickx G. F., van Heukelom L. H., Staal G. E., de Bree P. K., Wadman S. K., Ballieux R. E. Purine nucleoside phosphorylase deficiency associated with selective cellular immunodeficiency. N Engl J Med. 1977 Mar 24;296(12):651–655. doi: 10.1056/NEJM197703242961203. [DOI] [PubMed] [Google Scholar]
- Strobel S., Morgan G., Simmonds A. H., Levinsky R. J. Fatal graft versus host disease after platelet transfusions in a child with purine nucleoside phosphorylase deficiency. Eur J Pediatr. 1989 Jan;148(4):312–314. doi: 10.1007/BF00444121. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Clift S. M., Martin D. W., Jr Isolation and characterization of purine-nucleoside phosphorylase-deficient T-lymphoma cells and secondary mutants with altered ribonucleotide reductase: genetic model for immunodeficiency disease. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1074–1078. doi: 10.1073/pnas.76.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Hamet M., Ballet J. J., Reinert P., Griscelli C. Impaired defense against vaccinia in a child with T-lymphocyte deficiency associated with inosine phosphorylase defect. J Pediatr. 1978 Mar;92(3):358–362. doi: 10.1016/s0022-3476(78)80419-3. [DOI] [PubMed] [Google Scholar]
- Wang L. M., Weber D. K., Johnson T., Sakaguchi A. Y. Supercoil sequencing using unpurified templates produced by rapid boiling. Biotechniques. 1988 Oct;6(9):839, 841-3. [PubMed] [Google Scholar]
- Watson A. R., Evans D. I., Marsden H. B., Miller V., Rogers P. A. Purine nucleoside phosphorylase deficiency associated with a fatal lymphoproliferative disorder. Arch Dis Child. 1981 Jul;56(7):563–565. doi: 10.1136/adc.56.7.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Williams S. R., Gekeler V., McIvor R. S., Martin D. W., Jr A human purine nucleoside phosphorylase deficiency caused by a single base change. J Biol Chem. 1987 Feb 15;262(5):2332–2338. [PubMed] [Google Scholar]
- Williams S. R., Goddard J. M., Martin D. W., Jr Human purine nucleoside phosphorylase cDNA sequence and genomic clone characterization. Nucleic Acids Res. 1984 Jul 25;12(14):5779–5787. doi: 10.1093/nar/12.14.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann R. L., Andres C., Kaminska J., Mejias E., Gelfand E., Arnold W., Rich K., Fox I. H. Purine nucleoside phosphorylase deficiency: biochemical properties and heterogeneity in two families. Arthritis Rheum. 1979 May;22(5):524–531. doi: 10.1002/art.1780220513. [DOI] [PubMed] [Google Scholar]
- Zannis V., Doyle D., Martin D. W., Jr Purification and characterization of human erythrocyte purine nucleoside phosphorylase and its subunits. J Biol Chem. 1978 Jan 25;253(2):504–510. [PubMed] [Google Scholar]
- van Heukelom L. H., Staal G. E., Stoop J. W., Zegers B. J. An abnormal form of purine nucleoside phosphorylase in a family with a child with severe defective T-cell-and normal B-cell immunity. Clin Chim Acta. 1976 Oct 1;72(1):117–124. doi: 10.1016/0009-8981(76)90042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]