Abstract
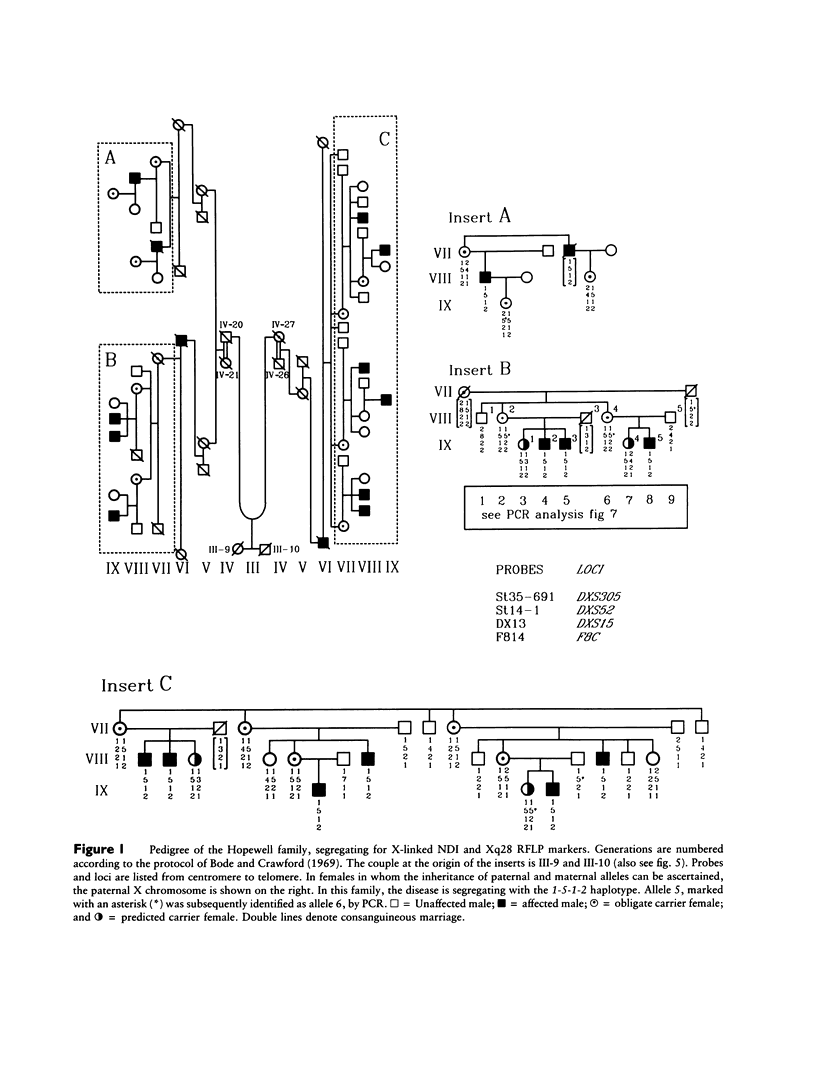
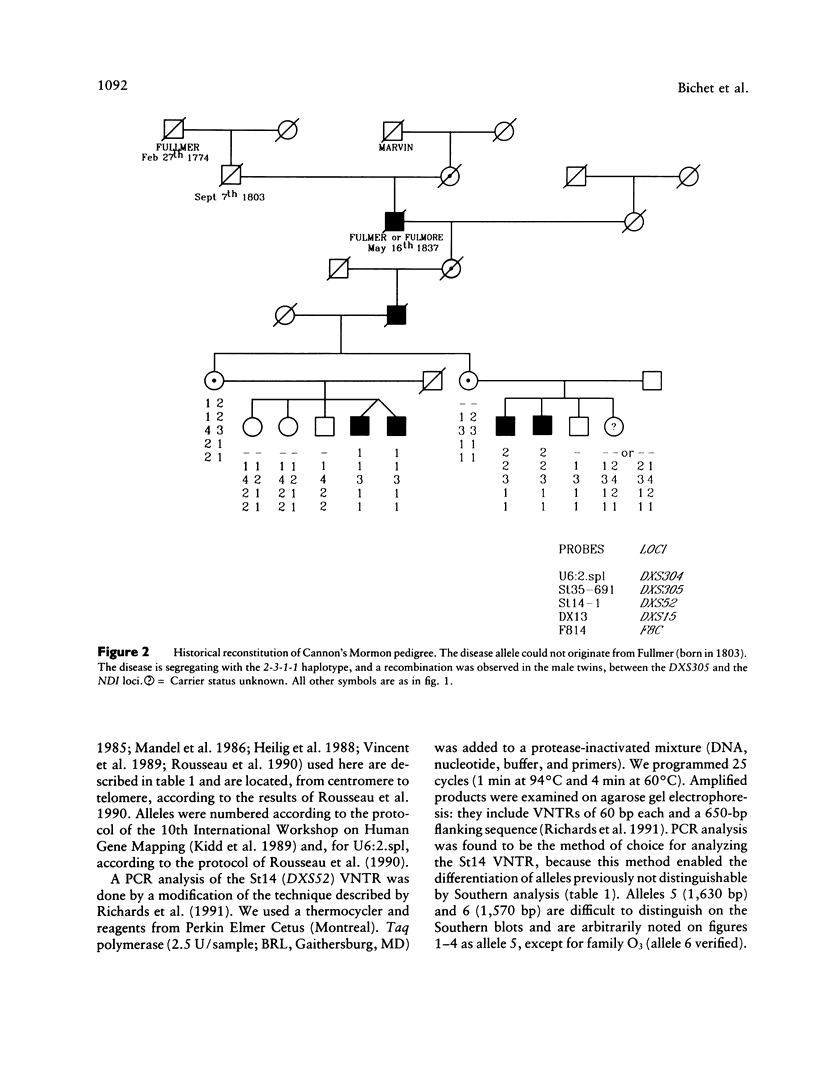
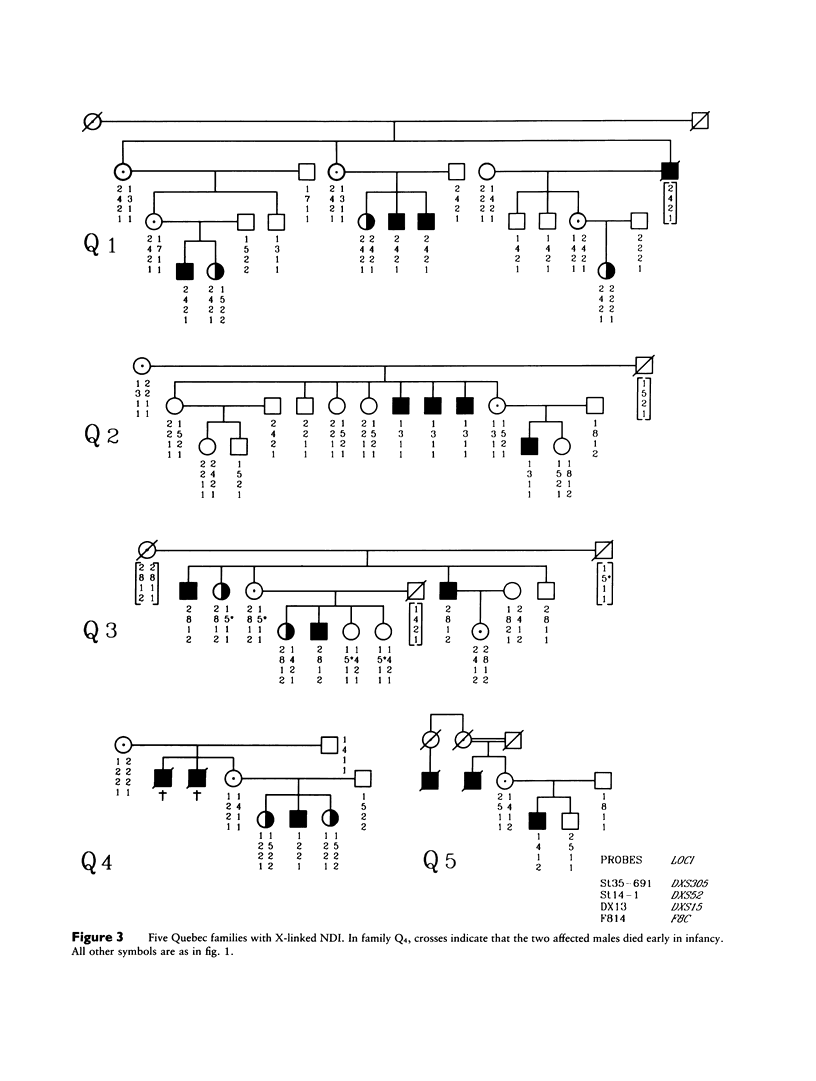
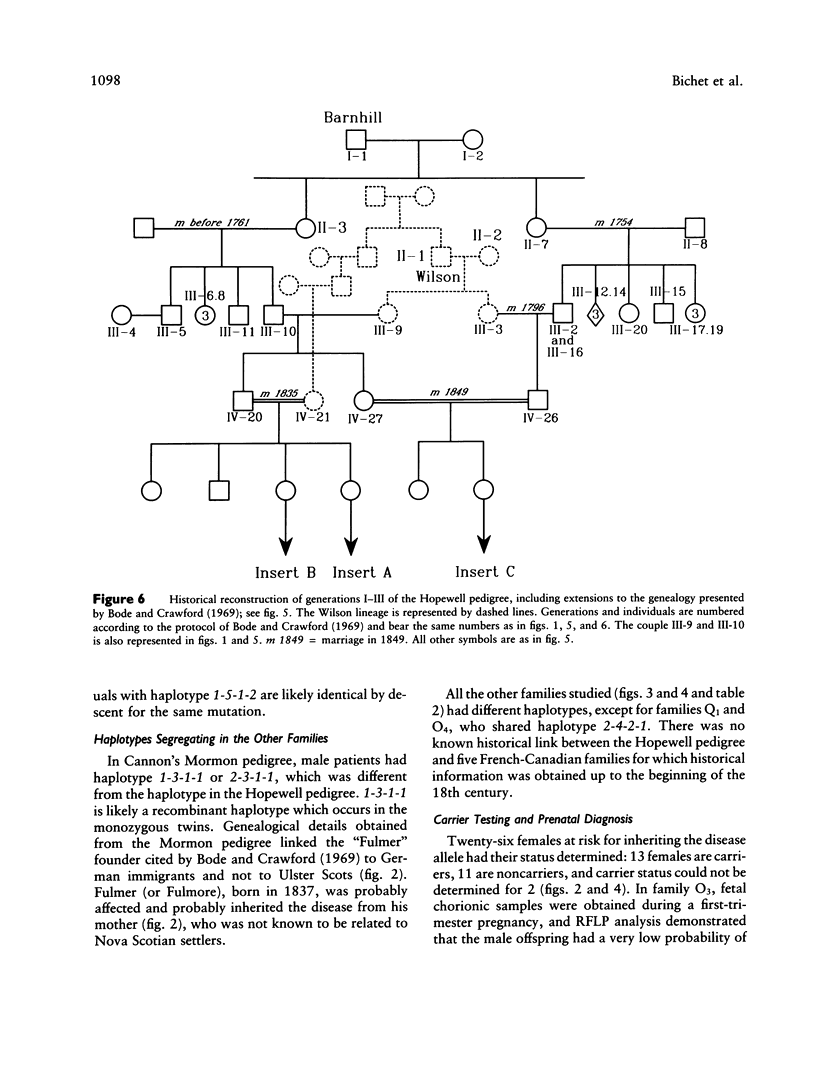
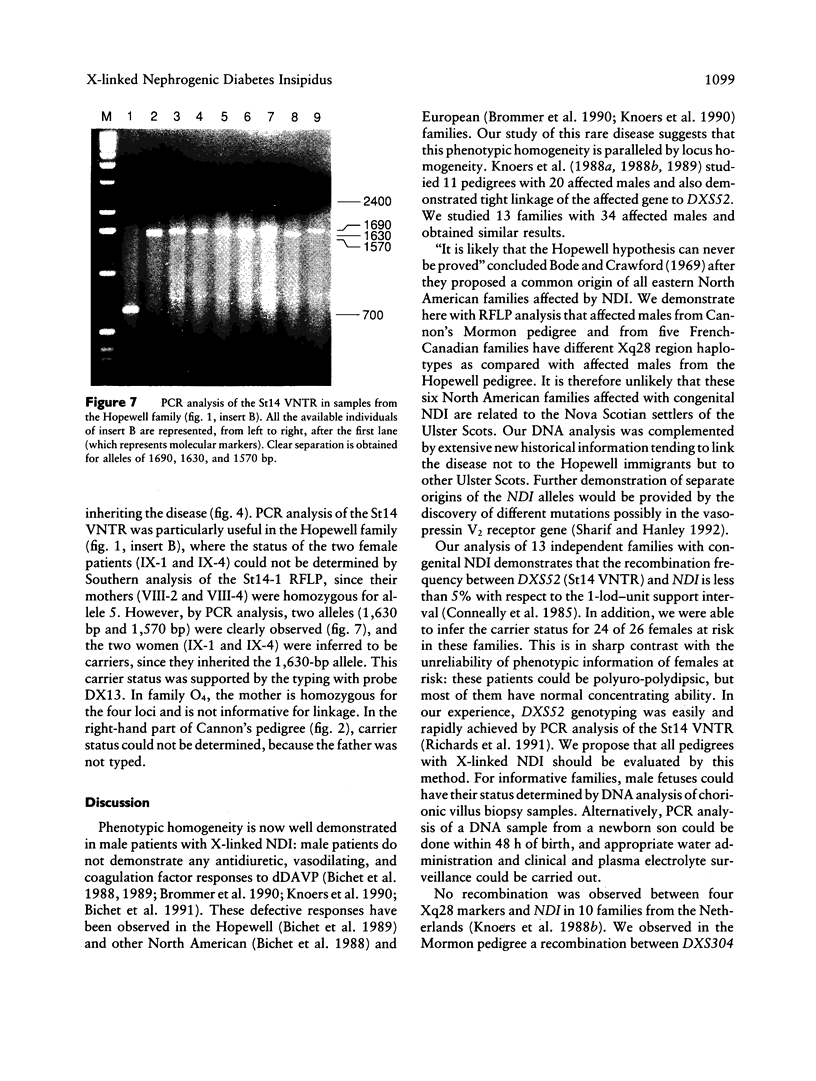
Nephrogenic diabetes insipidus (NDI; designated 304800 in Mendelian Inheritance in Man) is an X-linked disorder with abnormal renal and extrarenal V2 vasopressin receptor responses. The mutant gene has been mapped to Xq28 by analysis of RFLPs, and tight linkage between DXS52 and NDI has been reported. In 1969, Bode and Crawford proposed, under the term “the Hopewell hypothesis,” that most cases in North America could be traced to descendants of Ulster Scots who arrived in Nova Scotia in 1761 on the ship Hopewell. They also suggested a link between this family and a large Mormon pedigree. DNA samples obtained from 13 independent affected families, including 42 members of the Hopewell and Mormon pedigrees, were analyzed with probes in the Xq28 region. Genealogical reconstructions were performed. Linkage between NDI and DXS304 (probe U6:2.spl), DXS305 (St35-691), DXS52 (St14-1), DXS15 (DX13), and F8C (F814) showed no recombination in 12 families, with a maximum lod score of 13.5 for DXS52. A recombinant between NDI and DXS304, DXS305, was identified in one family. The haplotype segregating with the disease in the Hopewell pedigree was not shared by other North American families. PCR analysis of the St14 VNTR allowed the distinction of two alleles that were not distinguishable by Southern analysis. Carrier status was predicted in 24 of 26 at-risk females. The Hopewell hypothesis cannot explain the origin of NDI in many of the North American families, since they have no apparent relationship with the Hopewell early settlers, either by haplotype or by genealogical analysis. We confirm the locus homogeneity of the disease by linkage analysis in ethnically diverse families. PCR analysis of the DXS52 VNTR in NDI families is very useful for carrier testing and presymptomatic diagnosis, which can prevent the first manifestations of dehydration.
Full text
PDF

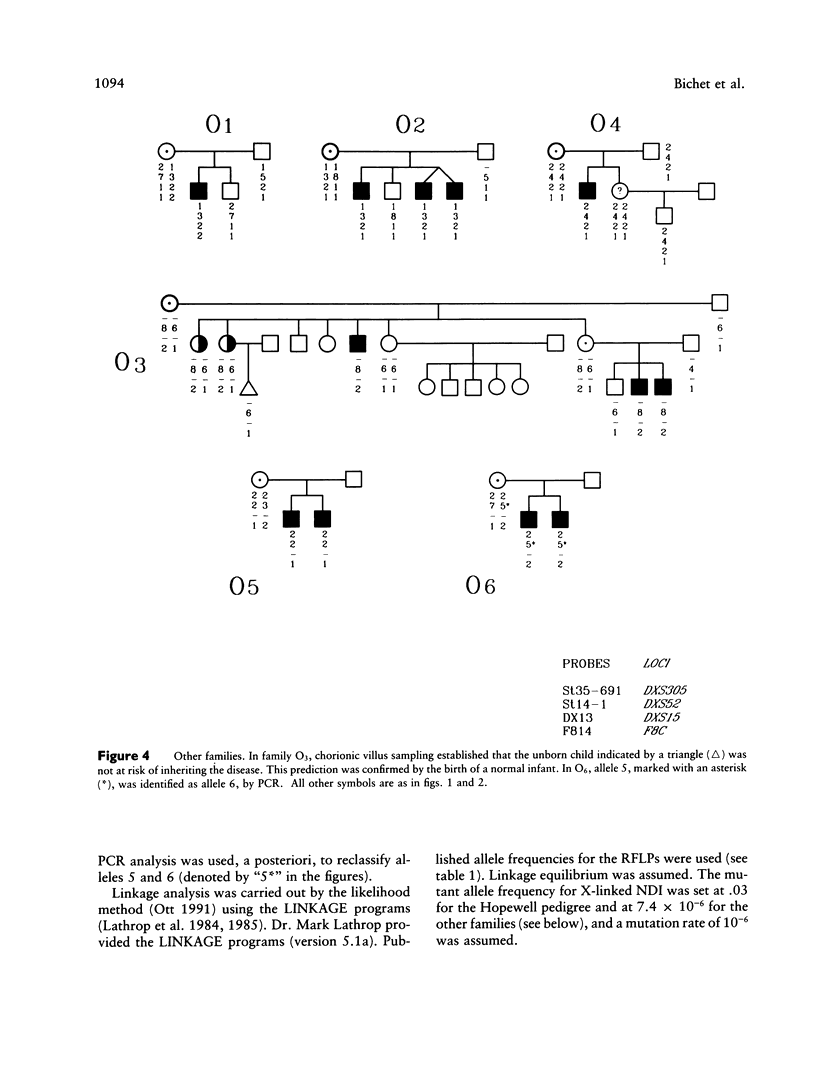
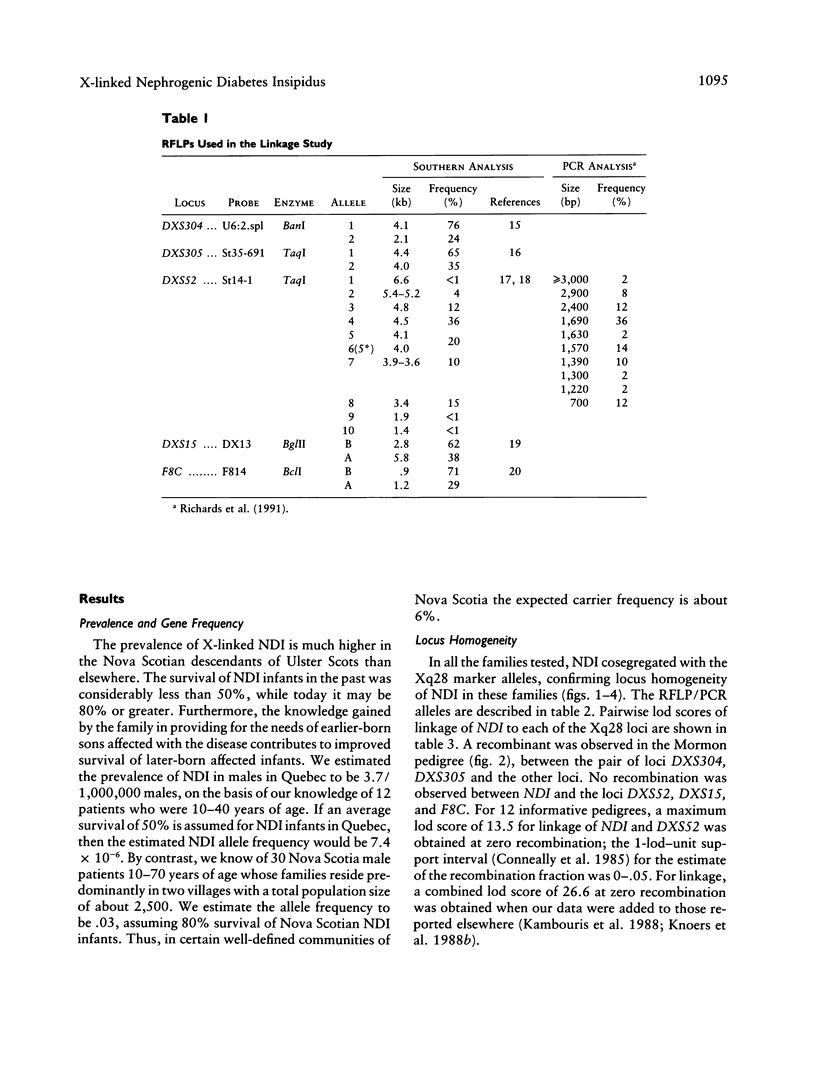
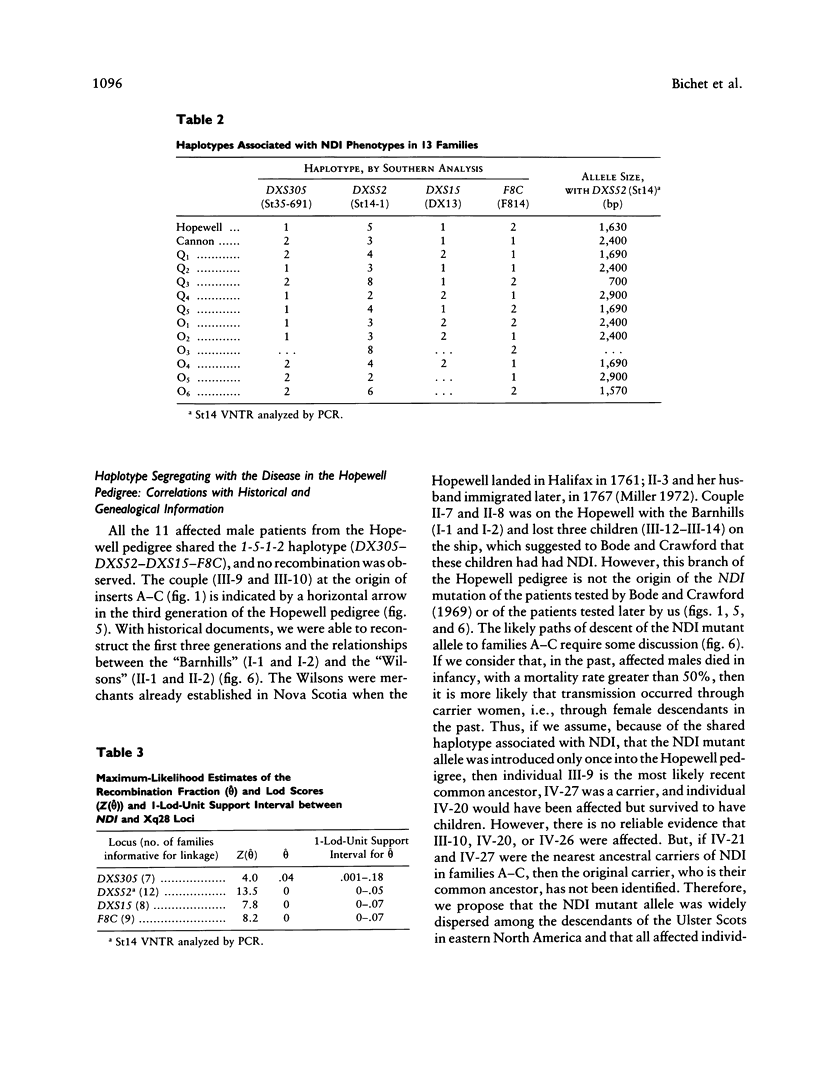
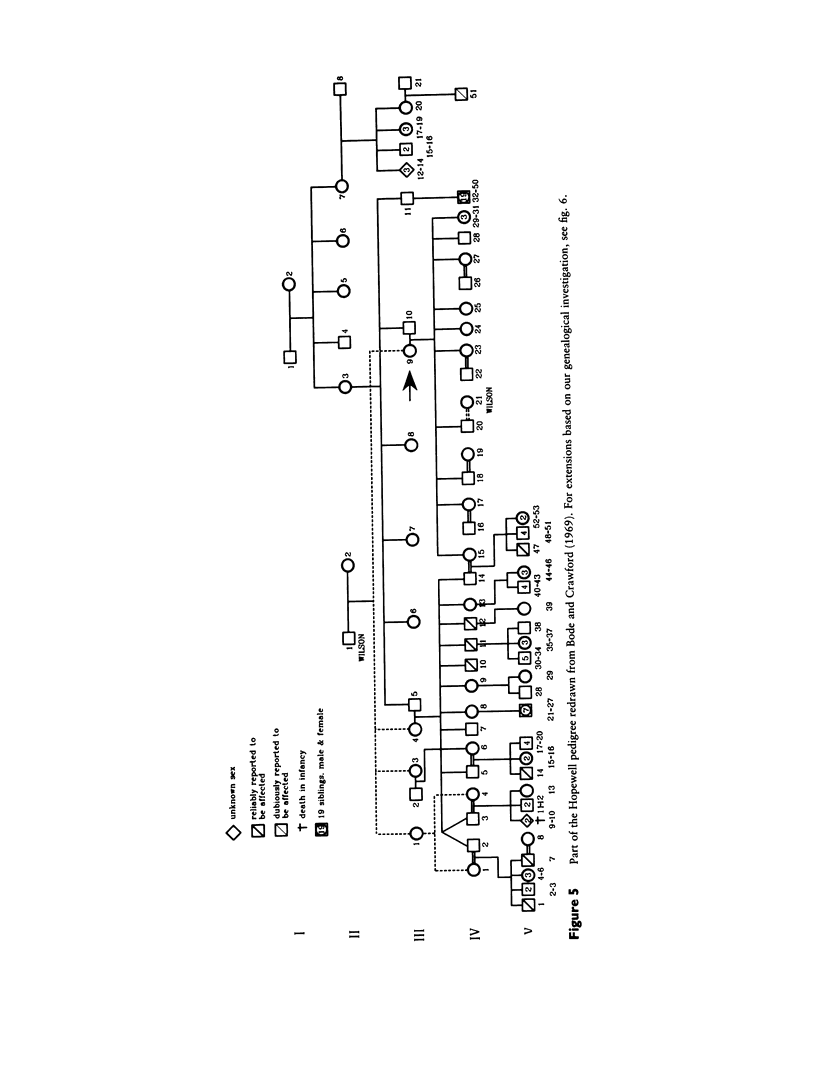



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bichet D. G., Arthus M. F., Lonergan M. Platelet vasopressin receptors in patients with congenital nephrogenic diabetes insipidus. Kidney Int. 1991 Apr;39(4):693–699. doi: 10.1038/ki.1991.83. [DOI] [PubMed] [Google Scholar]
- Bichet D. G., Razi M., Arthus M. F., Lonergan M., Tittley P., Smiley R. K., Rock G., Hirsch D. J. Epinephrine and dDAVP administration in patients with congenital nephrogenic diabetes insipidus. Evidence for a pre-cyclic AMP V2 receptor defective mechanism. Kidney Int. 1989 Nov;36(5):859–866. doi: 10.1038/ki.1989.272. [DOI] [PubMed] [Google Scholar]
- Bichet D. G., Razi M., Lonergan M., Arthus M. F., Papukna V., Kortas C., Barjon J. N. Hemodynamic and coagulation responses to 1-desamino[8-D-arginine] vasopressin in patients with congenital nephrogenic diabetes insipidus. N Engl J Med. 1988 Apr 7;318(14):881–887. doi: 10.1056/NEJM198804073181403. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M., Seibold A., Gilbert S., Ishido M., Barberis C., Antaramian A., Brabet P., Rosenthal W. Molecular cloning of the receptor for human antidiuretic hormone. Nature. 1992 May 28;357(6376):333–335. doi: 10.1038/357333a0. [DOI] [PubMed] [Google Scholar]
- Bode H. H., Crawford J. D. Nephrogenic diabetes insipidus in North America. The Hopewell hypothesis. N Engl J Med. 1969 Apr 3;280(14):750–754. doi: 10.1056/NEJM196904032801404. [DOI] [PubMed] [Google Scholar]
- Brommer E. J., Brink H., Derkx F. H., Schalekamp M. A., Stibbe J. Normal homeostasis of fibrinolysis in nephrogenic diabetes insipidus in spite of defective V2- receptor-mediated responses of tissue plasminogen activator release. Eur J Clin Invest. 1990 Feb;20(1):72–78. doi: 10.1111/j.1365-2362.1990.tb01794.x. [DOI] [PubMed] [Google Scholar]
- CANNON J. F. Diabetes insipidus; clinical and experimental studies with consideration of genetic relationships. AMA Arch Intern Med. 1955 Aug;96(2):215–272. doi: 10.1001/archinte.1955.00250130089012. [DOI] [PubMed] [Google Scholar]
- Conneally P. M., Edwards J. H., Kidd K. K., Lalouel J. M., Morton N. E., Ott J., White R. Report of the Committee on Methods of Linkage Analysis and Reporting. Cytogenet Cell Genet. 1985;40(1-4):356–359. doi: 10.1159/000132186. [DOI] [PubMed] [Google Scholar]
- Feil R., Palmieri G., d'Urso M., Heilig R., Oberlé I., Mandel J. L. Physical and genetic mapping of polymorphic loci in Xq28 (DXS15, DXS52, and DXS134): analysis of a cosmid clone and a yeast artificial chromosome. Am J Hum Genet. 1990 Apr;46(4):720–728. [PMC free article] [PubMed] [Google Scholar]
- Harper K., Winter R. M., Pembrey M. E., Hartley D., Davies K. E., Tuddenham E. G. A clinically useful DNA probe closely linked to haemophilia A. Lancet. 1984 Jul 7;2(8393):6–8. doi: 10.1016/s0140-6736(84)91995-0. [DOI] [PubMed] [Google Scholar]
- Heilig R., Oberlé I., Arveiler B., Hanauer A., Vidaud M., Mandel J. L. Improved DNA markers for efficient analysis of fragile X families. Am J Med Genet. 1988 May-Jun;30(1-2):543–550. doi: 10.1002/ajmg.1320300156. [DOI] [PubMed] [Google Scholar]
- Jans D. A., van Oost B. A., Ropers H. H., Fahrenholz F. Derivatives of somatic cell hybrids which carry the human gene locus for nephrogenic diabetes insipidus (NDI) express functional vasopressin renal V2-type receptors. J Biol Chem. 1990 Sep 15;265(26):15379–15382. [PubMed] [Google Scholar]
- Kambouris M., Dlouhy S. R., Trofatter J. A., Conneally P. M., Hodes M. E. Localization of the gene for X-linked nephrogenic diabetes insipidus to Xq28. Am J Med Genet. 1988 Jan;29(1):239–246. doi: 10.1002/ajmg.1320290138. [DOI] [PubMed] [Google Scholar]
- Kidd K. K., Bowcock A. M., Schmidtke J., Track R. K., Ricciuti F., Hutchings G., Bale A., Pearson P., Willard H. F., Gelernter J. Report of the DNA committee and catalogs of cloned and mapped genes and DNA polymorphisms. Cytogenet Cell Genet. 1989;51(1-4):622–947. doi: 10.1159/000132810. [DOI] [PubMed] [Google Scholar]
- Knoers N., Brommer E. J., Willems H., van Oost B. A., Monnens L. A. Fibrinolytic responses to 1-desamino-8-D-arginine-vasopressin in patients with congenital nephrogenic diabetes insipidus. Nephron. 1990;54(4):322–326. doi: 10.1159/000185888. [DOI] [PubMed] [Google Scholar]
- Knoers N., vd Heyden H., von Oost B. A., Monnens L., Willems J., Ropers H. H. Linkage of X-linked nephrogenic diabetes insipidus with DXS52, a polymorphic DNA marker. Nephron. 1988;50(3):187–190. doi: 10.1159/000185155. [DOI] [PubMed] [Google Scholar]
- Kobrinsky N. L., Doyle J. J., Israels E. D., Winter J. S., Cheang M. S., Walker R. D., Bishop A. J. Absent factor VIII response to synthetic vasopressin analogue (DDAVP) in nephrogenic diabetes insipidus. Lancet. 1985 Jun 8;1(8441):1293–1294. doi: 10.1016/s0140-6736(85)92790-4. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait S. J., O'Carroll A. M., McBride O. W., Konig M., Morel A., Brownstein M. J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992 May 28;357(6376):336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Arveiler B., Camerino G., Hanauer A., Heilig R., Koenig M., Oberlé I. Genetic mapping of the human X chromosome: linkage analysis of the q26-q28 region that includes the fragile X locus and isolation of expressed sequences. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):195–203. doi: 10.1101/sqb.1986.051.01.024. [DOI] [PubMed] [Google Scholar]
- Mulligan L. M., Grover H. J., Blanchette V. S., Giles A. R., Lillicrap D. P., Phillips A., Holden J. J., White B. N. Recombination between the factor VIII gene and the DXS52 locus gives the most probable genetic order as centromere-fra(X)-DXS15-DXS52-F8C-telomere. Am J Med Genet. 1987 Mar;26(3):751–760. doi: 10.1002/ajmg.1320260334. [DOI] [PubMed] [Google Scholar]
- Oberle I., Camerino G., Heilig R., Grunebaum L., Cazenave J. P., Crapanzano C., Mannucci P. M., Mandel J. L. Genetic screening for hemophilia A (classic hemophilia) with a polymorphic DNA probe. N Engl J Med. 1985 Mar 14;312(11):682–686. doi: 10.1056/NEJM198503143121103. [DOI] [PubMed] [Google Scholar]
- Poustka A., Dietrich A., Langenstein G., Toniolo D., Warren S. T., Lehrach H. Physical map of human Xq27-qter: localizing the region of the fragile X mutation. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8302–8306. doi: 10.1073/pnas.88.19.8302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards B., Heilig R., Oberlé I., Storjohann L., Horn G. T. Rapid PCR analysis of the St14 (DXS52) VNTR. Nucleic Acids Res. 1991 Apr 25;19(8):1944–1944. doi: 10.1093/nar/19.8.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau F., Vincent A., Oberlé I., Mandel J. L. New informative polymorphism at the DXS304 locus, a close distal marker for the fragile X locus. Hum Genet. 1990 Feb;84(3):263–266. doi: 10.1007/BF00200572. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Little R. D., Freije D., Abidi F., Zucchi I., Porta G., Pilia G., Nagaraja R., Johnson S. K., Yoon J. Y. Yeast artificial chromosome-based genome mapping: some lessons from Xq24-q28. Genomics. 1991 Dec;11(4):783–793. doi: 10.1016/0888-7543(91)90001-u. [DOI] [PubMed] [Google Scholar]
- Sharif M., Hanley M. R. Peptide receptors. Stepping up the pressure. Nature. 1992 May 28;357(6376):279–280. doi: 10.1038/357279a0. [DOI] [PubMed] [Google Scholar]
- Suthers G. K., Oberlé I., Nancarrow J., Mulley J. C., Hyland V. J., Wilson P. J., McCure J., Morris C. P., Hopwood J. J., Mandel J. L. Genetic mapping of new RFLPs at Xq27-q28. Genomics. 1991 Jan;9(1):37–43. doi: 10.1016/0888-7543(91)90218-4. [DOI] [PubMed] [Google Scholar]
- Vincent A., Kretz C., Oberlé I., Mandel J. L. A new polymorphic marker very closely linked to DXS52 in the q28 region of the human X chromosome. Hum Genet. 1989 Apr;82(1):85–86. doi: 10.1007/BF00288280. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., Knoop M. T., Knoers V. V., Markslag P. W., Rocchi M., Warren S. T., Ropers H. H., Fahrenholz F., Monnens L. A., van Oost B. A. Colocalization of the gene for nephrogenic diabetes insipidus (DIR) and the vasopressin type 2 receptor gene (AVPR2) in the Xq28 region. Genomics. 1992 Aug;13(4):1350–1352. doi: 10.1016/0888-7543(92)90067-3. [DOI] [PubMed] [Google Scholar]