Abstract
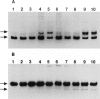
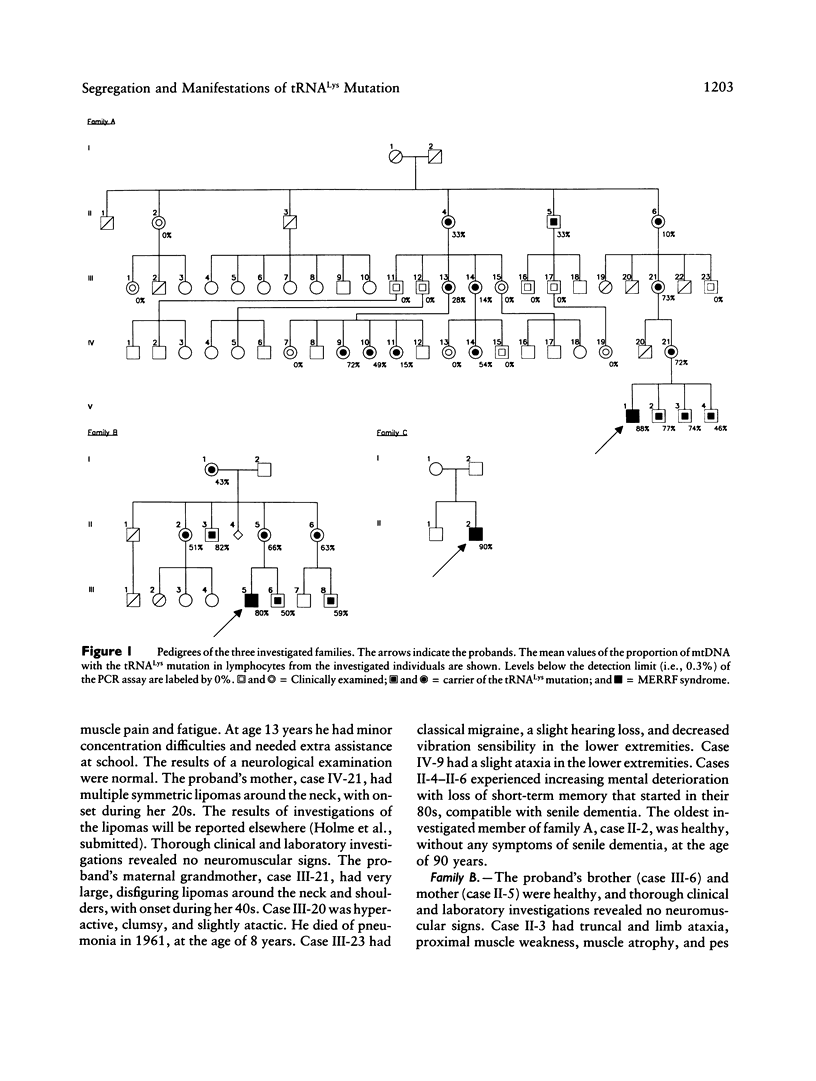
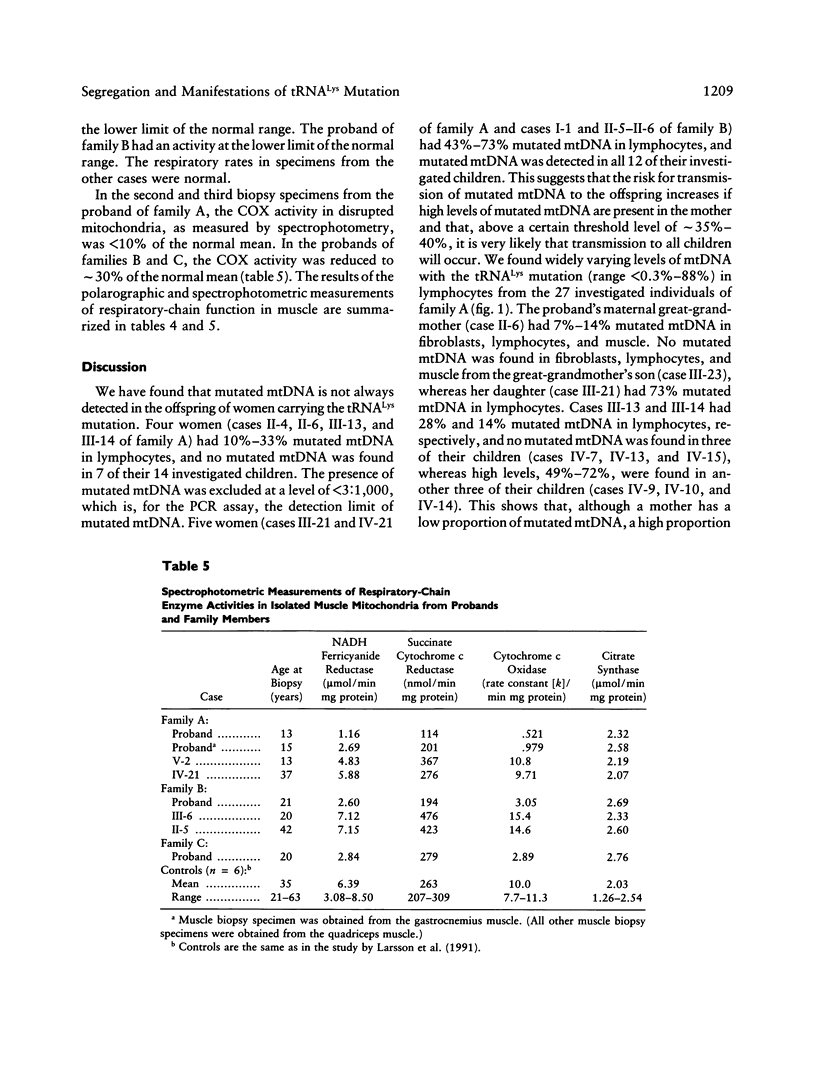
We have studied the segregation and manifestations of the tRNA(Lys) A-->G(8344) mutation of mtDNA. Three unrelated patients with myoclonus epilepsy and ragged-red fibers (MERRF) syndrome were investigated, along with 30 of their maternal relatives. Mutated mtDNA was not always found in the offspring of women carrying the tRNA(Lys) mutation. Four women had 10%-33% of mutated mtDNA in lymphocytes, and no mutated mtDNA was found in 7 of their 14 investigated children. The presence of mutated mtDNA was excluded at a level of 3:1,000. Five women had a proportion of 43%-73% mutated mtDNA in lymphocytes, and mutated mtDNA was found in all their 12 investigated children. This suggests that the risk for transmission of mutated mtDNA to the offspring increases if high levels are present in the mother and that, above a threshold level of 35%-40%, it is very likely that transmission will occur to all children. The three patients with MERRF syndrome had, in muscle, both 94%-96% mutated mtDNA and biochemical and histochemical evidence of a respiratory-chain dysfunction. Four relatives had a proportion of 61%-92% mutated mtDNA in muscle, and biochemical measurements showed a normal respiratory-chain function in muscle in all cases. These findings suggest that > 92% of mtDNA with the tRNA(Lys) mutation in muscle is required to cause a respiratory-chain dysfunction that can be detected by biochemical methods. There was a positive correlation between the levels of mtDNA with the tRNA(Lys) mutation in lymphocytes and the levels in muscle, in all nine investigated cases. The levels of mutated mtDNA were higher in muscle than in lymphocytes in all cases. In two of the patients with MERRF syndrome, muscle specimens were obtained at different times. In both cases, biochemical measurements revealed a deteriorating respiratory-chain function, and in one case a progressive increase in the amount of cytochrome c oxidase-deficient muscle fibers was found.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Ashley M. V., Laipis P. J., Hauswirth W. W. Rapid segregation of heteroplasmic bovine mitochondria. Nucleic Acids Res. 1989 Sep 25;17(18):7325–7331. doi: 10.1093/nar/17.18.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkovic S. F., Carpenter S., Evans A., Karpati G., Shoubridge E. A., Andermann F., Meyer E., Tyler J. L., Diksic M., Arnold D. Myoclonus epilepsy and ragged-red fibres (MERRF). 1. A clinical, pathological, biochemical, magnetic resonance spectrographic and positron emission tomographic study. Brain. 1989 Oct;112(Pt 5):1231–1260. doi: 10.1093/brain/112.5.1231. [DOI] [PubMed] [Google Scholar]
- Berkovic S. F., Shoubridge E. A., Andermann F., Andermann E., Carpenter S., Karpati G. Clinical spectrum of mitochondrial DNA mutation at base pair 8344. Lancet. 1991 Aug 17;338(8764):457–457. doi: 10.1016/0140-6736(91)91090-h. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Tokiguchi S., Shirakawa K., Tsubaki T. Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities ): disease entity or a syndrome? Light-and electron-microscopic studies of two cases and review of literature. J Neurol Sci. 1980 Jul;47(1):117–133. doi: 10.1016/0022-510x(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Giles R. E., Blanc H., Cann H. M., Wallace D. C. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Larsson N. G., Andersen O., Holme E., Oldfors A., Wahlström J. Leber's hereditary optic neuropathy and complex I deficiency in muscle. Ann Neurol. 1991 Nov;30(5):701–708. doi: 10.1002/ana.410300511. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Lombes A., Mendell J. R., Nakase H., Barohn R. J., Bonilla E., Zeviani M., Yates A. J., Omerza J., Gales T. L., Nakahara K. Myoclonic epilepsy and ragged-red fibers with cytochrome oxidase deficiency: neuropathology, biochemistry, and molecular genetics. Ann Neurol. 1989 Jul;26(1):20–33. doi: 10.1002/ana.410260104. [DOI] [PubMed] [Google Scholar]
- Mita S., Schmidt B., Schon E. A., DiMauro S., Bonilla E. Detection of "deleted" mitochondrial genomes in cytochrome-c oxidase-deficient muscle fibers of a patient with Kearns-Sayre syndrome. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9509–9513. doi: 10.1073/pnas.86.23.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noer A. S., Sudoyo H., Lertrit P., Thyagarajan D., Utthanaphol P., Kapsa R., Byrne E., Marzuki S. A tRNA(Lys) mutation in the mtDNA is the causal genetic lesion underlying myoclonic epilepsy and ragged-red fiber (MERRF) syndrome. Am J Hum Genet. 1991 Oct;49(4):715–722. [PMC free article] [PubMed] [Google Scholar]
- Oldfors A., Larsson N. G., Holme E., Tulinius M., Kadenbach B., Droste M. Mitochondrial DNA deletions and cytochrome c oxidase deficiency in muscle fibres. J Neurol Sci. 1992 Jul;110(1-2):169–177. doi: 10.1016/0022-510x(92)90025-g. [DOI] [PubMed] [Google Scholar]
- Rosing H. S., Hopkins L. C., Wallace D. C., Epstein C. M., Weidenheim K. Maternally inherited mitochondrial myopathy and myoclonic epilepsy. Ann Neurol. 1985 Mar;17(3):228–237. doi: 10.1002/ana.410170303. [DOI] [PubMed] [Google Scholar]
- Seibel P., Degoul F., Bonne G., Romero N., François D., Paturneau-Jouas M., Ziegler F., Eymard B., Fardeau M., Marsac C. Genetic biochemical and pathophysiological characterization of a familial mitochondrial encephalomyopathy (MERRF). J Neurol Sci. 1991 Oct;105(2):217–224. doi: 10.1016/0022-510x(91)90148-z. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Tanno Y., Yoneda M., Nonaka I., Tanaka K., Miyatake T., Tsuji S. Quantitation of mitochondrial DNA carrying tRNALys mutation in MERRF patients. Biochem Biophys Res Commun. 1991 Sep 16;179(2):880–885. doi: 10.1016/0006-291x(91)91900-w. [DOI] [PubMed] [Google Scholar]
- Tulinius M. H., Holme E., Kristiansson B., Larsson N. G., Oldfors A. Mitochondrial encephalomyopathies in childhood. I. Biochemical and morphologic investigations. J Pediatr. 1991 Aug;119(2):242–250. doi: 10.1016/s0022-3476(05)80734-6. [DOI] [PubMed] [Google Scholar]
- Vilkki J., Savontaus M. L., Nikoskelainen E. K. Segregation of mitochondrial genomes in a heteroplasmic lineage with Leber hereditary optic neuroretinopathy. Am J Hum Genet. 1990 Jul;47(1):95–100. [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Zheng X. X., Lott M. T., Shoffner J. M., Hodge J. A., Kelley R. I., Epstein C. M., Hopkins L. C. Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of a mitochondrial DNA disease. Cell. 1988 Nov 18;55(4):601–610. doi: 10.1016/0092-8674(88)90218-8. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Zeviani M., Amati P., Bresolin N., Antozzi C., Piccolo G., Toscano A., DiDonato S. Rapid detection of the A----G(8344) mutation of mtDNA in Italian families with myoclonus epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1991 Feb;48(2):203–211. [PMC free article] [PubMed] [Google Scholar]