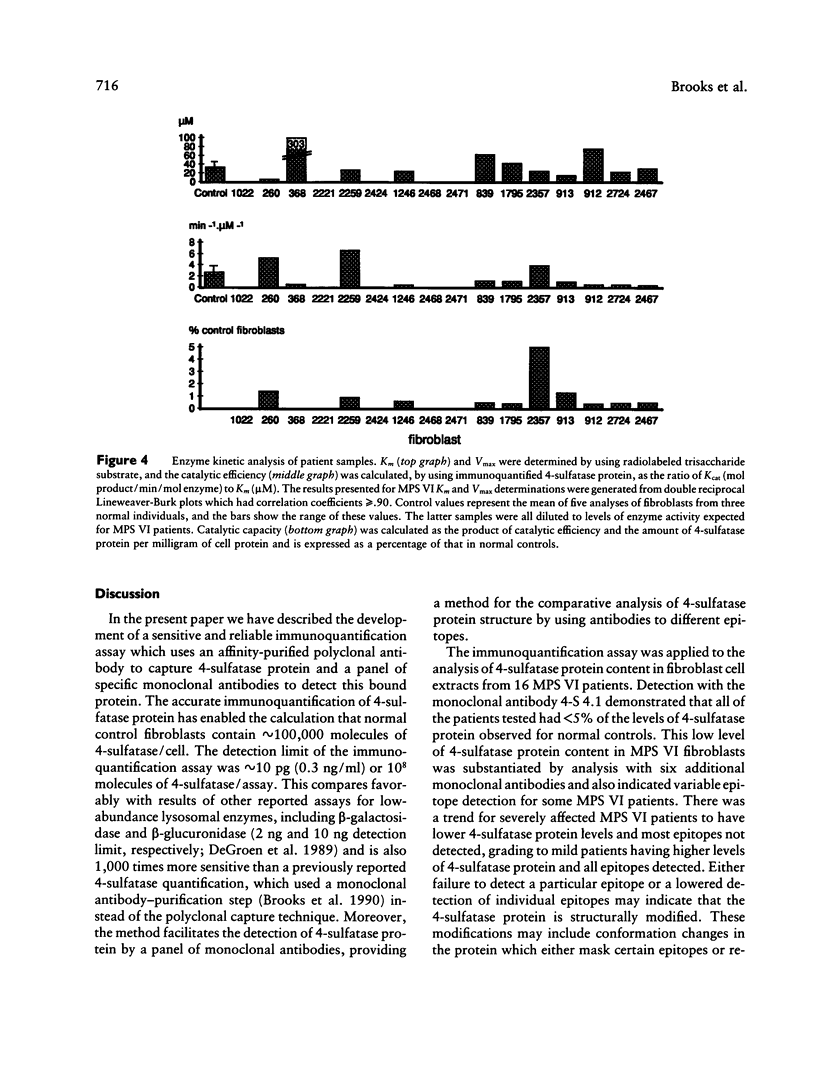
Abstract
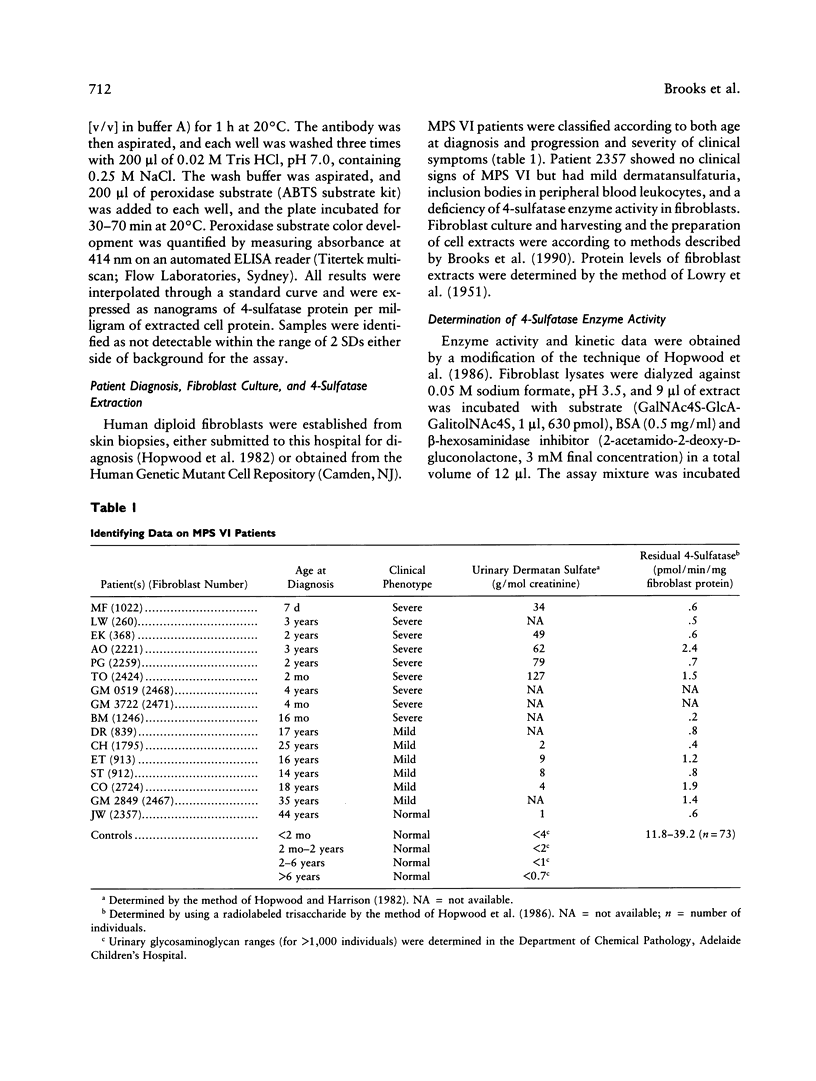
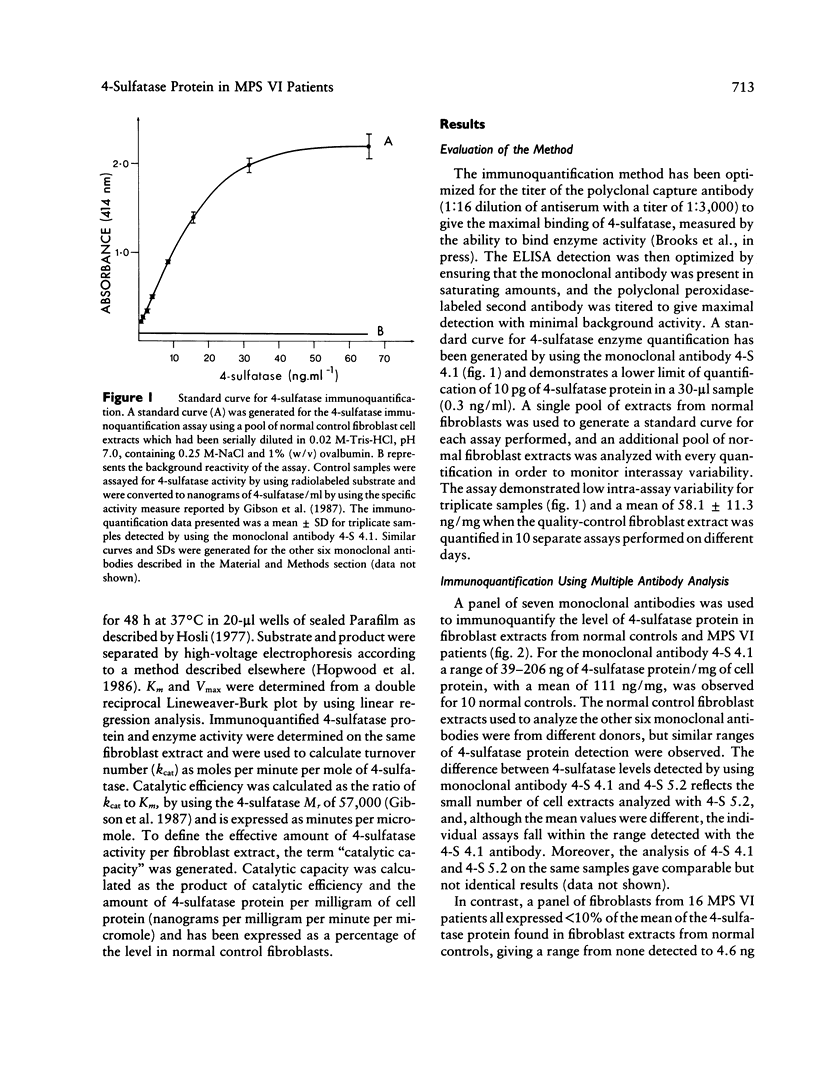
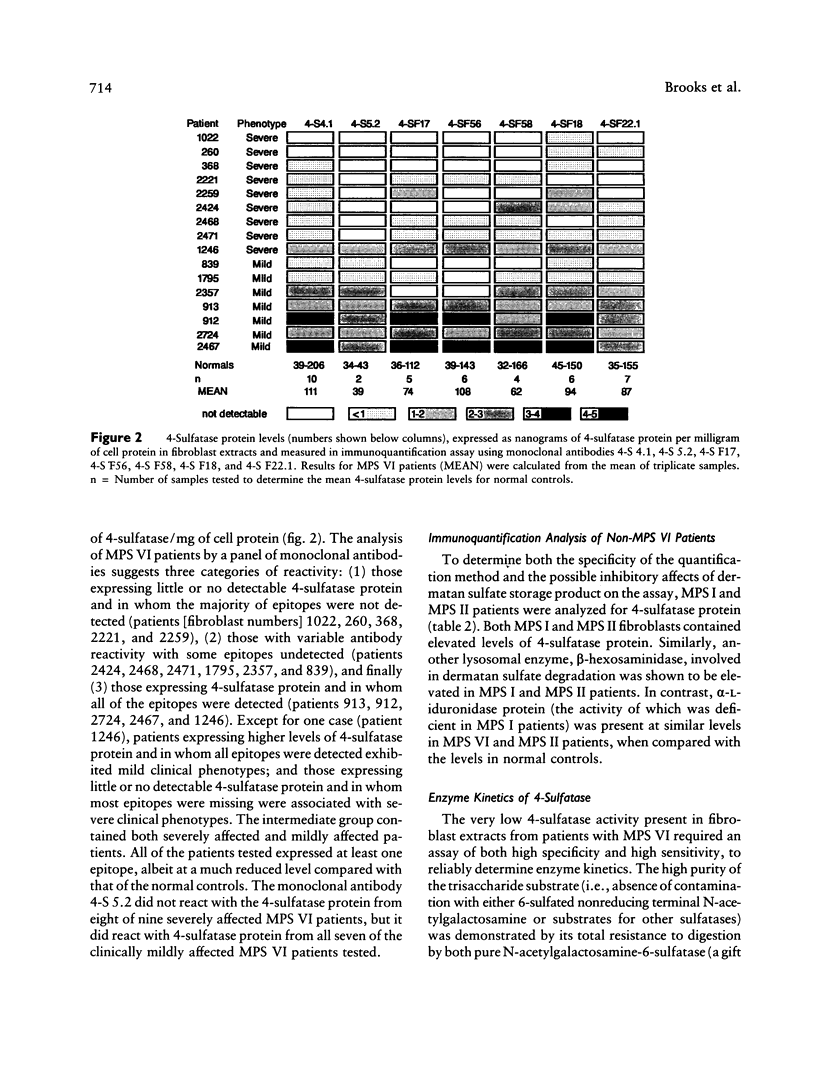
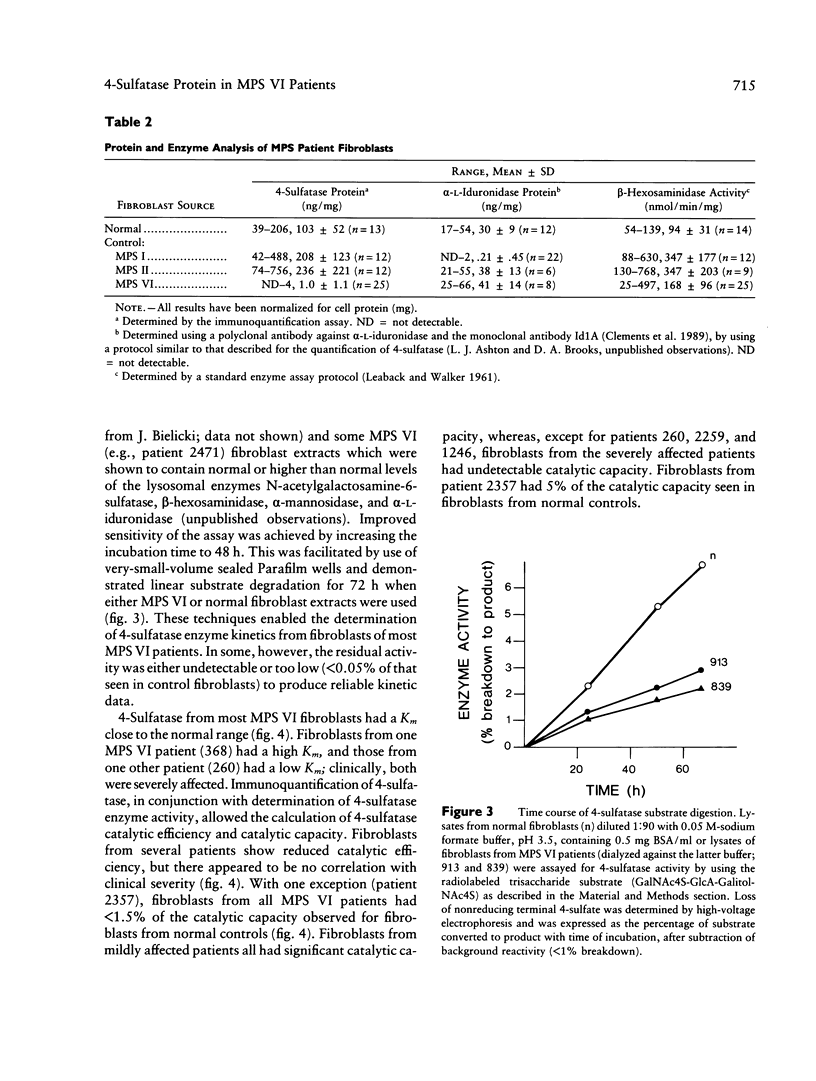
A sensitive and specific, monoclonal antibody-based immunoquantification assay has facilitated determination of the N-acetylgalactosamine-4-sulfatase (4-sulfatase) protein content in cultured fibroblasts from normal controls and mucopolysaccharidosis type VI (MPS VI) patients. The assay enabled the quantification of 4-sulfatase protein by using a panel of seven monoclonal antibodies and has shown that fibroblasts from 16 MPS VI patients contained less than or equal to 5% of the level determined for normal controls. Fibroblasts from the most severely affected patients contained the lowest levels of 4-sulfatase protein, usually with few epitopes detected, while fibroblasts from mildly affected patients had higher levels of 4-sulfatase protein, with all seven epitopes detected. The pattern of epitope expression is proposed to reflect the conformational changes in the 4-sulfatase protein that arise from different mutations in the 4-sulfatase gene. Immunoquantification in combination with a specific and highly sensitive 4-sulfated trisaccharide-based assay of enzyme activity in these MPS VI patient fibroblasts enabled the determination of residual 4-sulfatase catalytic efficiency (kcat/Km). The capacity of fibroblasts to degrade substrate (catalytic capacity) was calculated as the product of 4-sulfatase catalytic efficiency and the content of 4-sulfatase in fibroblasts. One patient, 2357, with no clinical signs of MPS VI but with reduced 4-sulfatase activity and protein (both 5% of normal) and dermatansulfaturia, had 5% of normal catalytic capacity. The other 15 MPS VI patient fibroblasts had 0%-1.4% of the catalytic capacity of fibroblasts from normal controls and were representative of the spectrum of MPS VI clinical phenotypes, from severe to mild.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks D. A., McCourt P. A., Gibson G. J., Hopwood J. J. Immunoquantification of the low abundance lysosomal enzyme N-acetylgalactosamine 4-sulphatase. J Inherit Metab Dis. 1990;13(1):108–120. doi: 10.1007/BF01799338. [DOI] [PubMed] [Google Scholar]
- Chang P. L., Rosa N. E., Davidson R. G. Differential assay of arylsulfatase A and B activities: a sensitive method for cultured human cells. Anal Biochem. 1981 Nov 1;117(2):382–389. doi: 10.1016/0003-2697(81)90795-8. [DOI] [PubMed] [Google Scholar]
- Clements P. R., Brooks D. A., McCourt P. A., Hopwood J. J. Immunopurification and characterization of human alpha-L-iduronidase with the use of monoclonal antibodies. Biochem J. 1989 Apr 1;259(1):199–208. doi: 10.1042/bj2590199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delvin E. E., Pottier A., Glorieux F. Comparative activity of arylsulphatases A and B on two synthetic substrates. Biochem J. 1976 Aug 1;157(2):353–356. doi: 10.1042/bj1570353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. J., Saccone G. T., Brooks D. A., Clements P. R., Hopwood J. J. Human N-acetylgalactosamine-4-sulphate sulphatase. Purification, monoclonal antibody production and native and subunit Mr values. Biochem J. 1987 Dec 15;248(3):755–764. doi: 10.1042/bj2480755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H., Muller V. J., Saccone G. T. Diagnosis of Maroteaux-Lamy syndrome by the use of radiolabelled oligosaccharides as substrates for the determination of arylsulphatase B activity. Biochem J. 1986 Mar 15;234(3):507–514. doi: 10.1042/bj2340507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood J. J., Harrison J. R. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982 Jan 1;119(1):120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- Hopwood J. J., Muller V., Harrison J. R., Carey W. F., Elliott H., Robertson E. F., Pollard A. C. Enzymatic diagnosis of the mucopolysaccharidoses: experience of 96 cases diagnosed in a five-year period. Med J Aust. 1982 Mar 20;1(6):257–260. [PubMed] [Google Scholar]
- Hösli P. Quantitative assays of enzyme activity in single cells: early prenatal diagnosis of genetic disorders. Clin Chem. 1977 Aug;23(8):1476–1484. [PubMed] [Google Scholar]
- LEABACK D. H., WALKER P. G. Studies on glucosaminidase. 4. The fluorimetric assay of N-acetyl-beta-glucosaminidase. Biochem J. 1961 Jan;78:151–156. doi: 10.1042/bj0780151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matalon R., Arbogast B., Dorfman A. Deficiency of chondroitin sulfate N-acetylgalactosamine 4-sulfate sulfatase in Maroteaux-Lamy syndrome. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1450–1457. doi: 10.1016/s0006-291x(74)80446-8. [DOI] [PubMed] [Google Scholar]
- Peters C., Schmidt B., Rommerskirch W., Rupp K., Zühlsdorf M., Vingron M., Meyer H. E., Pohlmann R., von Figura K. Phylogenetic conservation of arylsulfatases. cDNA cloning and expression of human arylsulfatase B. J Biol Chem. 1990 Feb 25;265(6):3374–3381. [PubMed] [Google Scholar]
- Rose J. K., Doms R. W. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Schuchman E. H., Jackson C. E., Desnick R. J. Human arylsulfatase B: MOPAC cloning, nucleotide sequence of a full-length cDNA, and regions of amino acid identity with arylsulfatases A and C. Genomics. 1990 Jan;6(1):149–158. doi: 10.1016/0888-7543(90)90460-c. [DOI] [PubMed] [Google Scholar]
- Taylor J. A., Gibson G. J., Brooks D. A., Hopwood J. J. Human N-acetylgalactosamine-4-sulphatase biosynthesis and maturation in normal, Maroteaux-Lamy and multiple-sulphatase-deficient fibroblasts. Biochem J. 1990 Jun 1;268(2):379–386. doi: 10.1042/bj2680379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groen P. C., LeSage G. D., Tietz P. S., LaRusso N. F. Purification and immunological quantification of rat liver lysosomal glycosidases. Biochem J. 1989 Nov 15;264(1):115–123. doi: 10.1042/bj2640115. [DOI] [PMC free article] [PubMed] [Google Scholar]


