Abstract
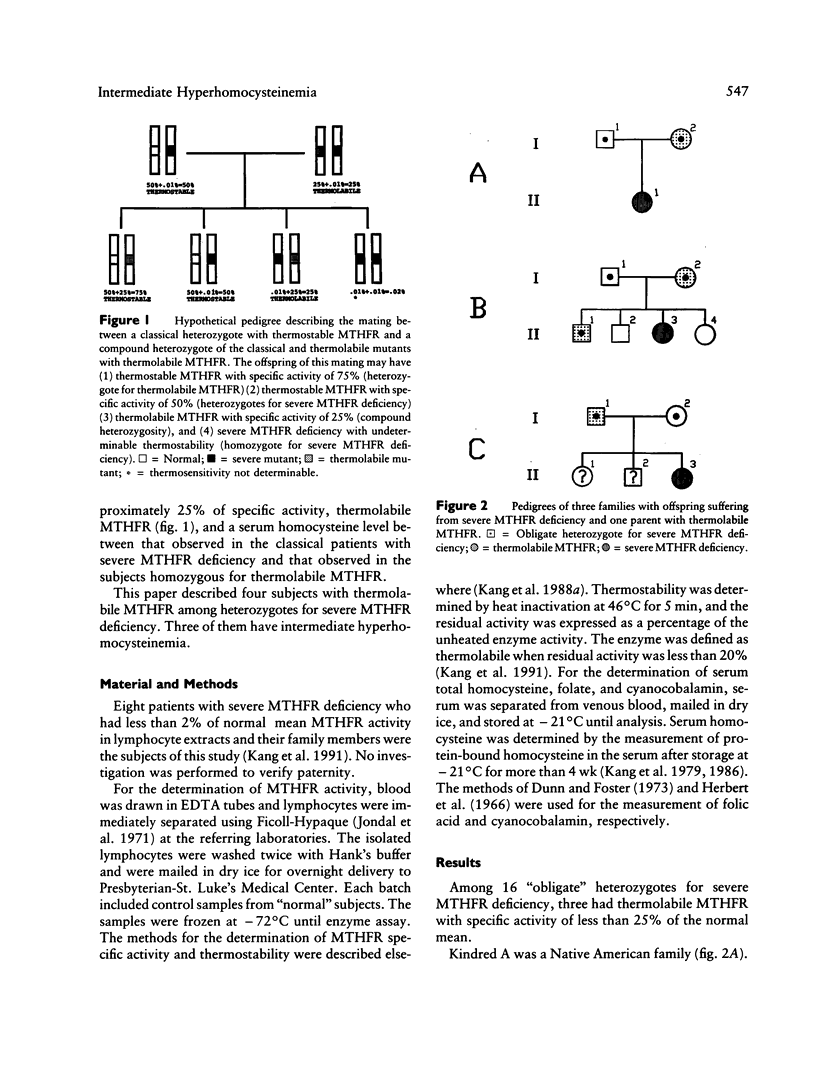
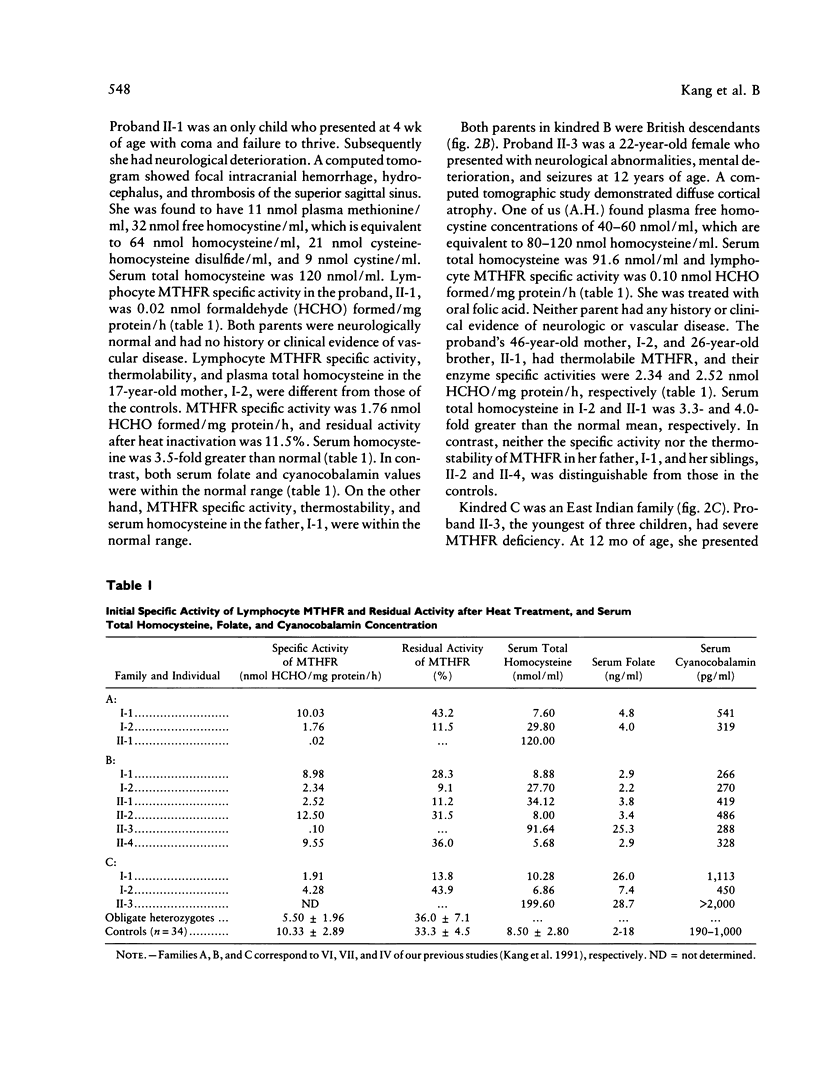
Four subjects with thermolabile methylenetetrahydrofolate reductase (MTHFR) were discovered among 16 "obligate" heterozygotes for severe MTHFR deficiency and their family members. All four subjects had less than 25% of normal mean MTHFR specific activity in lymphocyte extracts. Three of them with normal serum folate and cyanocobalamin had intermediate hyperhomocysteinemia, and one with high serum folate and cyanocobalamin had no excessive accumulation of serum homocysteine. The biochemical features in these four subjects are distinguishable from subjects homozygous for the thermolabile MTHFR, whose specific activity is approximately 50% of the normal mean, and from heterozygotes for severe MTHFR deficiency, in whom the enzyme is thermostable and has a specific activity of about 50% of the normal mean. We propose that these four subjects are genetic compounds of the allele for the severe mutation and the allele for thermolabile mutation of the MTHFR gene. It is postulated that subjects with this genetic compound are more susceptible to the development of intermediate hyperhomocysteinemia despite normal folate and B12 levels. Nonetheless, hyperhomocysteinemia due to this compound heterozygosity is correctable by oral folic acid therapy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dunn R. T., Foster L. B. Radioassay of serum folate. Clin Chem. 1973 Oct;19(10):1101–1105. [PubMed] [Google Scholar]
- Jondal M., Holm G., Wigzell H. Surface markers on human T and B lymphocytes. I. A large population of lymphocytes forming nonimmune rosettes with sheep red blood cells. J Exp Med. 1972 Aug 1;136(2):207–215. doi: 10.1084/jem.136.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Becker N. Protein-bound homocyst(e)ine in normal subjects and in patients with homocystinuria. Pediatr Res. 1979 Oct;13(10):1141–1143. doi: 10.1203/00006450-197910000-00012. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Norusis M. Homocysteinemia due to folate deficiency. Metabolism. 1987 May;36(5):458–462. doi: 10.1016/0026-0495(87)90043-6. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Susmano A., Sora J., Norusis M., Ruggie N. Thermolabile methylenetetrahydrofolate reductase: an inherited risk factor for coronary artery disease. Am J Hum Genet. 1991 Mar;48(3):536–545. [PMC free article] [PubMed] [Google Scholar]
- Kang S. S., Wong P. W., Zhou J. M., Sora J., Lessick M., Ruggie N., Grcevich G. Thermolabile methylenetetrahydrofolate reductase in patients with coronary artery disease. Metabolism. 1988 Jul;37(7):611–613. doi: 10.1016/0026-0495(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Kang S. S., Zhou J., Wong P. W., Kowalisyn J., Strokosch G. Intermediate homocysteinemia: a thermolabile variant of methylenetetrahydrofolate reductase. Am J Hum Genet. 1988 Oct;43(4):414–421. [PMC free article] [PubMed] [Google Scholar]
- McGill J. J., Mettler G., Rosenblatt D. S., Scriver C. R. Detection of heterozygotes for recessive alleles. Homocyst(e)inemia: paradigm of pitfalls in phenotypes. Am J Med Genet. 1990 May;36(1):45–52. doi: 10.1002/ajmg.1320360111. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Uhlendorf B. W., Freeman J. M., Finkelstein J. D., Shih V. E. Homocystinuria associated with decreased methylenetetrahydrofolate reductase activity. Biochem Biophys Res Commun. 1972 Jan 31;46(2):905–912. doi: 10.1016/s0006-291x(72)80227-4. [DOI] [PubMed] [Google Scholar]
- Murphy-Chutorian D. R., Wexman M. P., Grieco A. J., Heininger J. A., Glassman E., Gaull G. E., Ng S. K., Feit F., Wexman K., Fox A. C. Methionine intolerance: a possible risk factor for coronary artery disease. J Am Coll Cardiol. 1985 Oct;6(4):725–730. doi: 10.1016/s0735-1097(85)80473-3. [DOI] [PubMed] [Google Scholar]
- Sardharwalla I. B., Fowler B., Robins A. J., Komrower G. M. Detection of heterozygotes for homocystinuria. Study of sulphur-containing amino acids in plasma and urine after L-methionine loading. Arch Dis Child. 1974 Jul;49(7):553–559. doi: 10.1136/adc.49.7.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland P. M., Refsum H. Plasma homocysteine, a risk factor for vascular disease: plasma levels in health, disease, and drug therapy. J Lab Clin Med. 1989 Nov;114(5):473–501. [PubMed] [Google Scholar]
- Wilcken D. E., Reddy S. G., Gupta V. J. Homocysteinemia, ischemic heart disease, and the carrier state for homocystinuria. Metabolism. 1983 Apr;32(4):363–370. doi: 10.1016/0026-0495(83)90045-8. [DOI] [PubMed] [Google Scholar]
- Wong P. W., Justice P., Berlow S. Detection of homozygotes and heterozygotes with methylenetetrahydrofolate reductase deficiency. J Lab Clin Med. 1977 Aug;90(2):283–288. [PubMed] [Google Scholar]


