Abstract
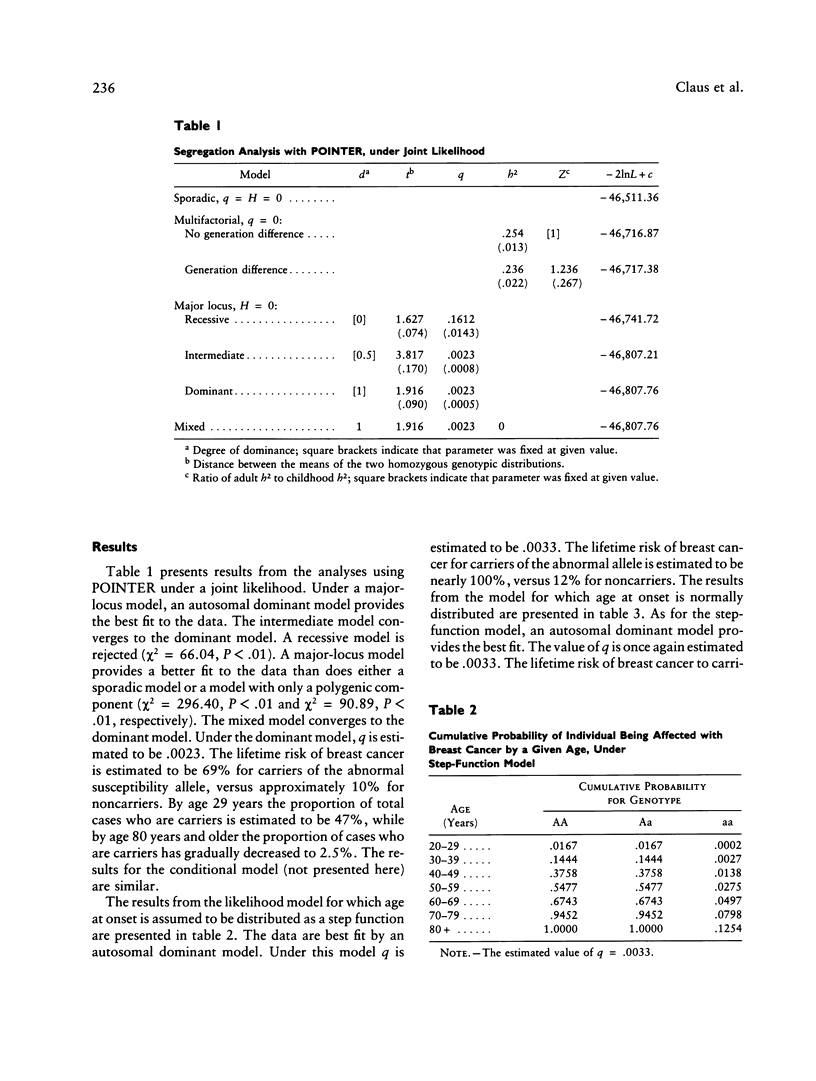
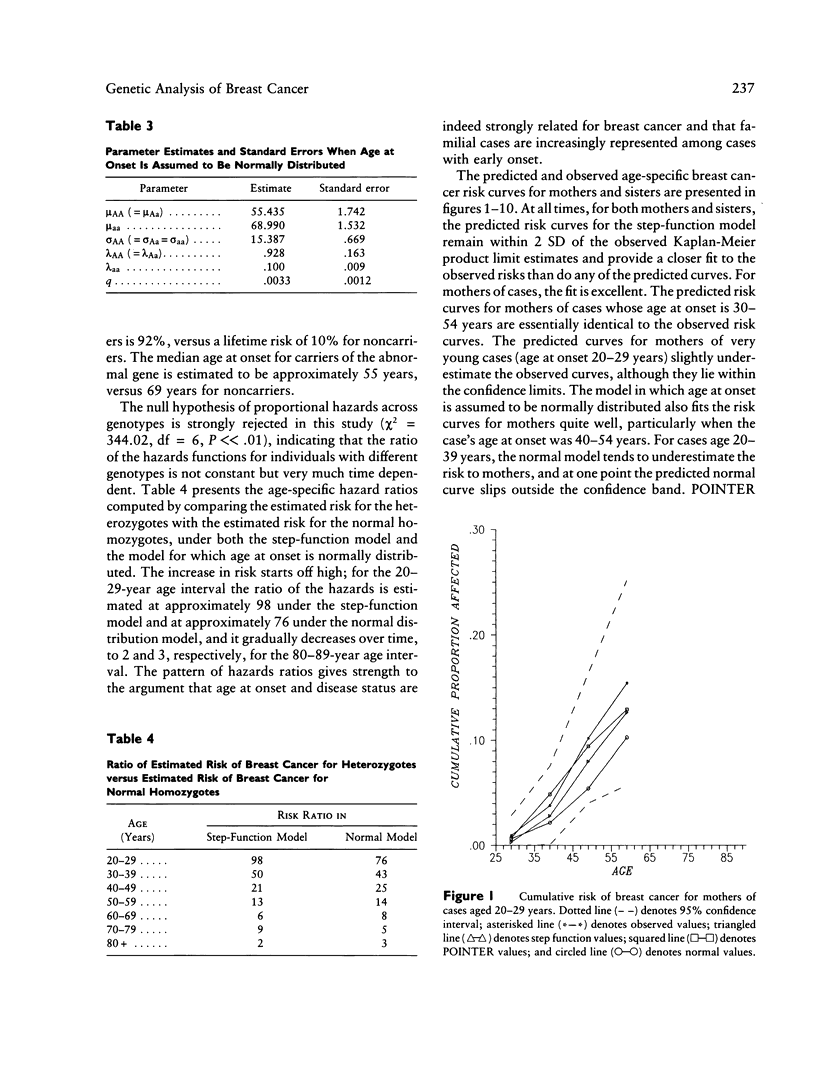
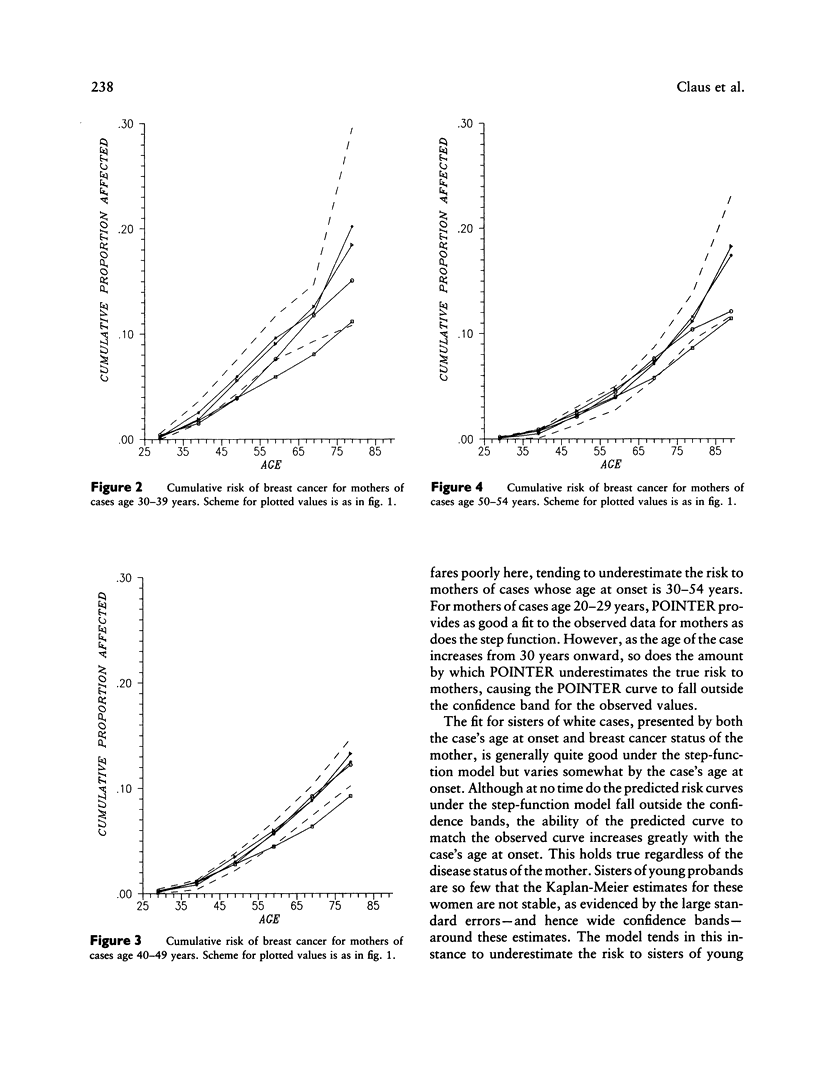
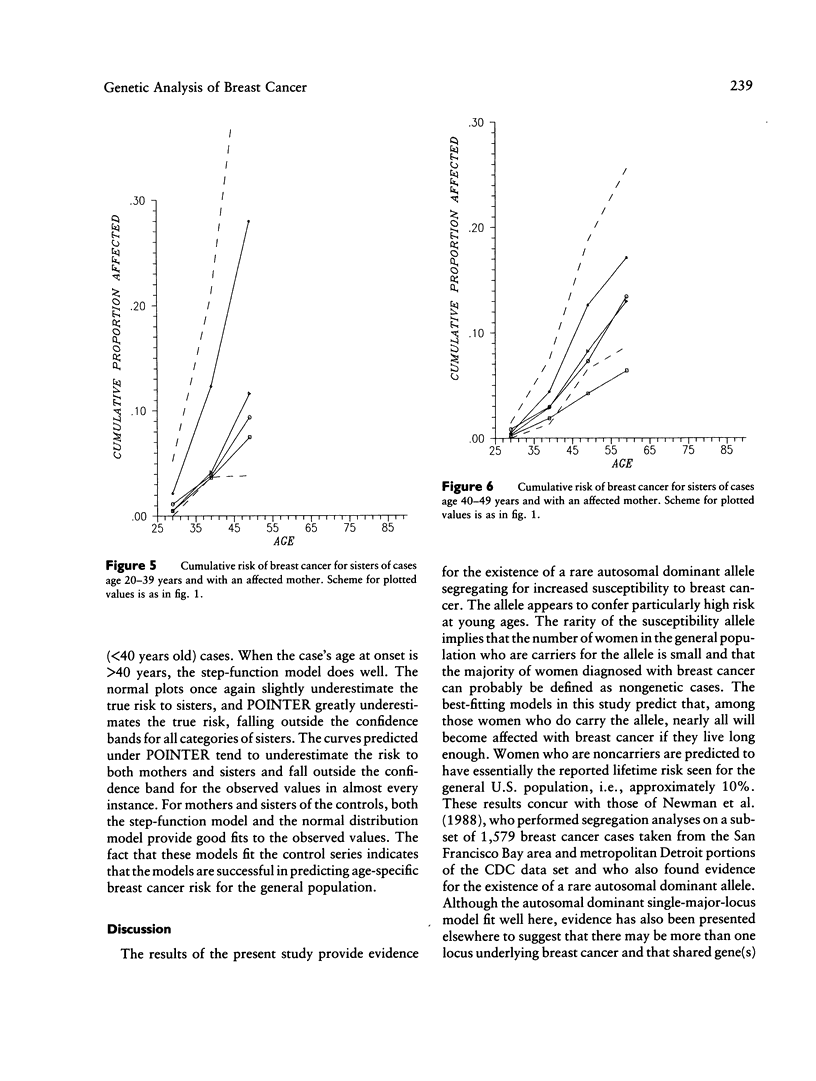
The familial risk of breast cancer is investigated in a large population-based, case-control study conducted by the Centers for Disease Control. The data set is based on 4,730 histologically confirmed breast cancer cases aged 20 to 54 years and on 4,688 controls who were frequency matched to cases on the basis of both geographic region and 5-year categories of age, and it includes family histories, obtained through interviews of cases and controls, of breast cancer in mothers and sisters. Segregation analysis and goodness-of-fit tests of genetic models provide evidence for the existence of a rare autosomal dominant allele (q = .0033) leading to increased susceptibility to breast cancer. The effect of genotype on the risk of breast cancer is shown to be a function of a woman's age. Although, compared with noncarriers, carriers of the allele appear to be at greater risk at all ages, the ratio of age-specific risks is greatest at young ages and declines steadily thereafter. The proportion of cases predicted to carry the allele is highest (36%) among cases aged 20-29 years. This proportion gradually decreases to 1% among cases aged 80 years or older. The cumulative lifetime risk of breast cancer for women who carry the susceptibility allele is predicted to be high, approximately 92%, while the cumulative lifetime risk for noncarriers is estimated to be approximately 10%.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. T., Cannon-Albright L., McLellan T., Gardner E. J., Skolnick M. H. Segregation and linkage analysis of nine Utah breast cancer pedigrees. Genet Epidemiol. 1988;5(3):151–169. doi: 10.1002/gepi.1370050303. [DOI] [PubMed] [Google Scholar]
- Claus E. B., Risch N. J., Thompson W. D. Age at onset as an indicator of familial risk of breast cancer. Am J Epidemiol. 1990 Jun;131(6):961–972. doi: 10.1093/oxfordjournals.aje.a115616. [DOI] [PubMed] [Google Scholar]
- Go R. C., King M. C., Bailey-Wilson J., Elston R. C., Lynch H. T. Genetic epidemiology of breast cancer and associated cancers in high-risk families. I. Segregation analysis. J Natl Cancer Inst. 1983 Sep;71(3):455–461. [PubMed] [Google Scholar]
- Goldstein A. M., Haile R. W., Hodge S. E., Paganini-Hill A., Spence M. A. Possible heterogeneity in the segregation pattern of breast cancer in families with bilateral breast cancer. Genet Epidemiol. 1988;5(2):121–133. doi: 10.1002/gepi.1370050207. [DOI] [PubMed] [Google Scholar]
- Goldstein A. M., Haile R. W., Marazita M. L., Paganini-Hill A. A genetic epidemiologic investigation of breast cancer in families with bilateral breast cancer. I. Segregation analysis. J Natl Cancer Inst. 1987 May;78(5):911–918. [PubMed] [Google Scholar]
- Lalouel J. M., Morton N. E. Complex segregation analysis with pointers. Hum Hered. 1981;31(5):312–321. doi: 10.1159/000153231. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Conway T., Fitzgibbons R., Jr, Schreiman J., Watson P., Marcus J., Fitzsimmons M. L., Lynch J. F. Age-of-onset heterogeneity in hereditary breast cancer: minimal clues for diagnosis. Breast Cancer Res Treat. 1988 Dec;12(3):275–285. doi: 10.1007/BF01811240. [DOI] [PubMed] [Google Scholar]
- Lynch H. T., Watson P., Conway T., Fitzsimmons M. L., Lynch J. Breast cancer family history as a risk factor for early onset breast cancer. Breast Cancer Res Treat. 1988 Jul;11(3):263–267. doi: 10.1007/BF01807285. [DOI] [PubMed] [Google Scholar]
- Sattin R. W., Rubin G. L., Webster L. A., Huezo C. M., Wingo P. A., Ory H. W., Layde P. M. Family history and the risk of breast cancer. JAMA. 1985 Apr 5;253(13):1908–1913. [PubMed] [Google Scholar]
- Schildkraut J. M., Risch N., Thompson W. D. Evaluating genetic association among ovarian, breast, and endometrial cancer: evidence for a breast/ovarian cancer relationship. Am J Hum Genet. 1989 Oct;45(4):521–529. [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. G., King M. C., Belle S. H., Satariano W. A., Swanson G. M. Risk of breast cancer to relatives of young breast cancer patients. J Natl Cancer Inst. 1985 Oct;75(4):665–668. [PubMed] [Google Scholar]
- Williams W. R., Anderson D. E. Genetic epidemiology of breast cancer: segregation analysis of 200 Danish pedigrees. Genet Epidemiol. 1984;1(1):7–20. doi: 10.1002/gepi.1370010104. [DOI] [PubMed] [Google Scholar]
- Wingo P. A., Ory H. W., Layde P. M., Lee N. C. The evaluation of the data collection process for a multicenter, population-based, case-control design. Am J Epidemiol. 1988 Jul;128(1):206–217. doi: 10.1093/oxfordjournals.aje.a114942. [DOI] [PubMed] [Google Scholar]


