Abstract
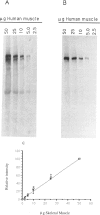
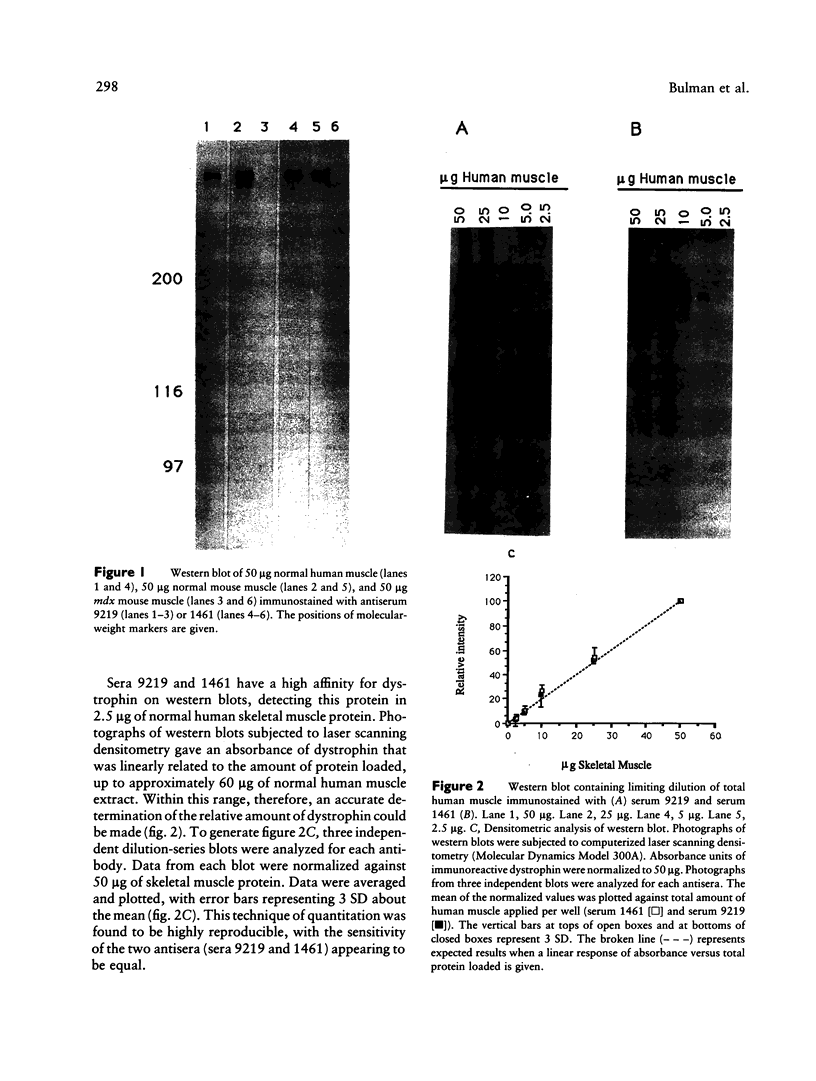
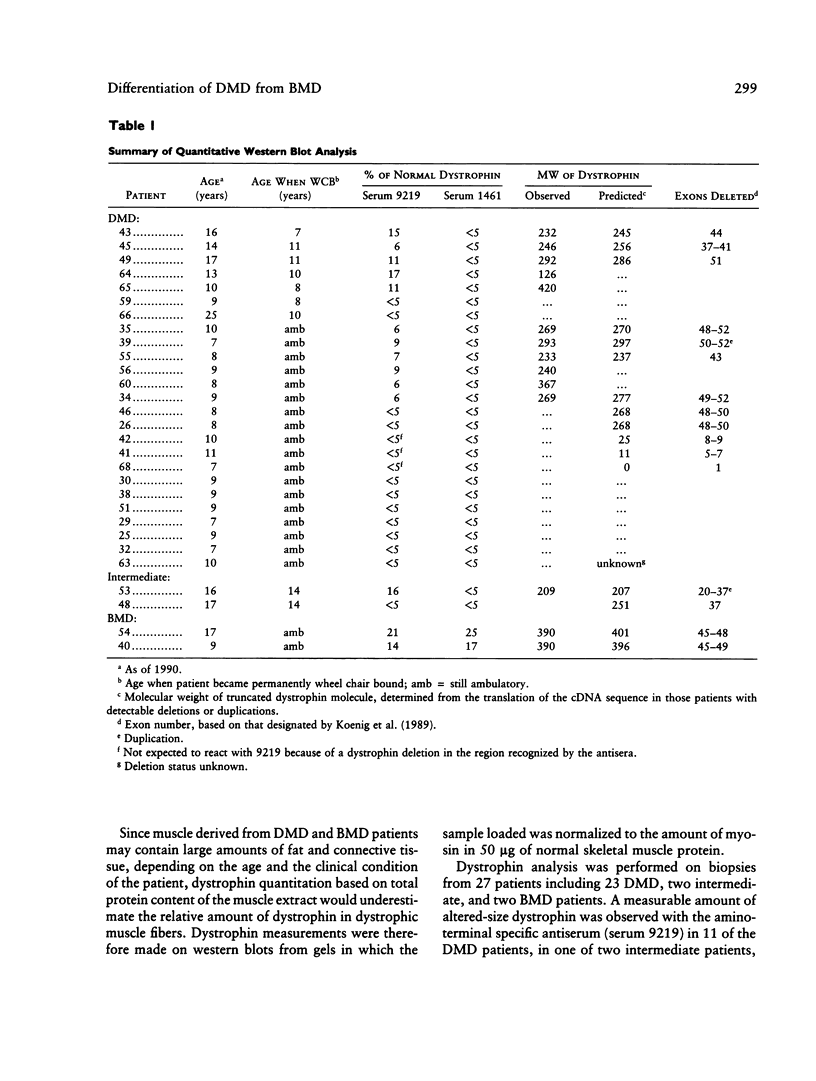
Antibodies directed against the amino- and carboxy-terminal regions of dystrophin have been used to characterize 25 Duchenne muscular dystrophy (DMD), two intermediate, and two Becker muscular dystrophy (BMD) patients. Western blot analysis revealed an altered-size (truncated) immunoreactive dystrophin band in 11 of the 25 DMD patients, in one of the two intermediate patients, and in both BMD patients, when immunostained with antiserum raised against the amino terminus of dystrophin. None of the DMD or intermediate patients demonstrated an immunoreactive dystrophin band when immunostained with an antiserum specific for the carboxy terminus of the protein. In contrast, dystrophin was detected in both BMD patients by the antiserum specific for the carboxy terminus. Quantitative studies indicated that the relative abundance of dystrophin in patients with a severe (DMD), intermediate, or mild (BMD) phenotype may overlap, therefore suggesting that differential diagnosis of disease severity based entirely on dystrophin quantitation may be unsatisfactory. Our results suggest that a differential diagnosis between DMD and BMD would benefit from examination of both the N terminus and C terminus of the protein, in addition to measurements of the relative abundance of the protein.
Full text
PDF



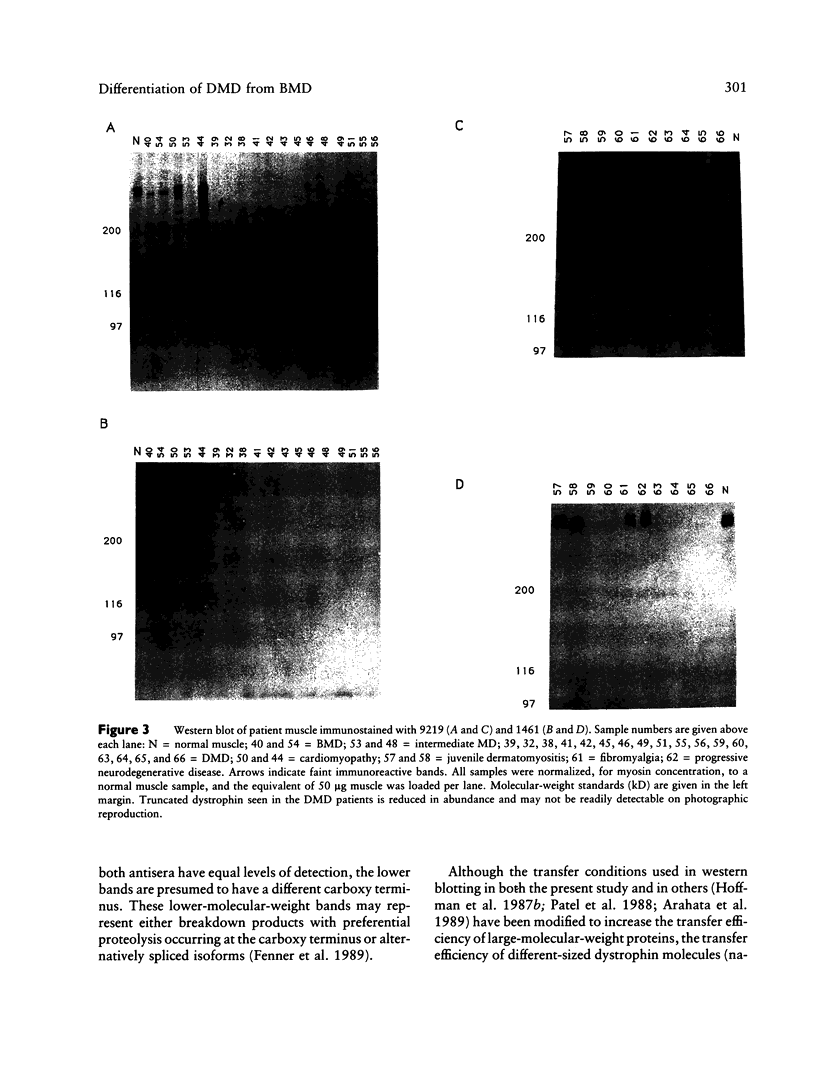



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arahata K., Hoffman E. P., Kunkel L. M., Ishiura S., Tsukahara T., Ishihara T., Sunohara N., Nonaka I., Ozawa E., Sugita H. Dystrophin diagnosis: comparison of dystrophin abnormalities by immunofluorescence and immunoblot analyses. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7154–7158. doi: 10.1073/pnas.86.18.7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K., Ishiura S., Ishiguro T., Tsukahara T., Suhara Y., Eguchi C., Ishihara T., Nonaka I., Ozawa E., Sugita H. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988 Jun 30;333(6176):861–863. doi: 10.1038/333861a0. [DOI] [PubMed] [Google Scholar]
- Baumbach L. L., Chamberlain J. S., Ward P. A., Farwell N. J., Caskey C. T. Molecular and clinical correlations of deletions leading to Duchenne and Becker muscular dystrophies. Neurology. 1989 Apr;39(4):465–474. doi: 10.1212/wnl.39.4.465. [DOI] [PubMed] [Google Scholar]
- Bonilla E., Samitt C. E., Miranda A. F., Hays A. P., Salviati G., DiMauro S., Kunkel L. M., Hoffman E. P., Rowland L. P. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988 Aug 12;54(4):447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- Burghes A. H., Logan C., Hu X., Belfall B., Worton R. G., Ray P. N. A cDNA clone from the Duchenne/Becker muscular dystrophy gene. 1987 Jul 30-Aug 5Nature. 328(6129):434–437. doi: 10.1038/328434a0. [DOI] [PubMed] [Google Scholar]
- Carpenter S., Karpati G., Zubrzycka-Gaarn E., Bulman D. E., Ray P. N., Worton R. G. Dystrophin is localized to the plasma membrane of human skeletal muscle fibers by electron-microscopic cytochemical study. Muscle Nerve. 1990 May;13(5):376–380. doi: 10.1002/mus.880130503. [DOI] [PubMed] [Google Scholar]
- Cullen M. J., Walsh J., Nicholson L. V., Harris J. B. Ultrastructural localization of dystrophin in human muscle by using gold immunolabelling. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):197–210. doi: 10.1098/rspb.1990.0034. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Critchley D. R. alpha-Actinins and the DMD protein contain spectrin-like repeats. Cell. 1988 Jan 29;52(2):159–160. doi: 10.1016/0092-8674(88)90503-x. [DOI] [PubMed] [Google Scholar]
- Forrest S. M., Cross G. S., Speer A., Gardner-Medwin D., Burn J., Davies K. E. Preferential deletion of exons in Duchenne and Becker muscular dystrophies. Nature. 1987 Oct 15;329(6140):638–640. doi: 10.1038/329638a0. [DOI] [PubMed] [Google Scholar]
- Gillard E. F., Chamberlain J. S., Murphy E. G., Duff C. L., Smith B., Burghes A. H., Thompson M. W., Sutherland J., Oss I., Bodrug S. E. Molecular and phenotypic analysis of patients with deletions within the deletion-rich region of the Duchenne muscular dystrophy (DMD) gene. Am J Hum Genet. 1989 Oct;45(4):507–520. [PMC free article] [PubMed] [Google Scholar]
- Hammonds R. G., Jr Protein sequence of DMD gene is related to actin-binding domain of alpha-actinin. Cell. 1987 Oct 9;51(1):1–1. doi: 10.1016/0092-8674(87)90002-x. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Brown R. H., Jr, Kunkel L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987 Dec 24;51(6):919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Fischbeck K. H., Brown R. H., Johnson M., Medori R., Loike J. D., Harris J. B., Waterston R., Brooke M., Specht L. Characterization of dystrophin in muscle-biopsy specimens from patients with Duchenne's or Becker's muscular dystrophy. N Engl J Med. 1988 May 26;318(21):1363–1368. doi: 10.1056/NEJM198805263182104. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Knudson C. M., Campbell K. P., Kunkel L. M. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987 Dec 24;330(6150):754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M., Angelini C., Clarke A., Johnson M., Harris J. B. Improved diagnosis of Becker muscular dystrophy by dystrophin testing. Neurology. 1989 Aug;39(8):1011–1017. doi: 10.1212/wnl.39.8.1011. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Kunkel L. M. Dystrophin abnormalities in Duchenne/Becker muscular dystrophy. Neuron. 1989 Jan;2(1):1019–1029. doi: 10.1016/0896-6273(89)90226-2. [DOI] [PubMed] [Google Scholar]
- Hu X. Y., Ray P. N., Murphy E. G., Thompson M. W., Worton R. G. Duplicational mutation at the Duchenne muscular dystrophy locus: its frequency, distribution, origin, and phenotypegenotype correlation. Am J Hum Genet. 1990 Apr;46(4):682–695. [PMC free article] [PubMed] [Google Scholar]
- Humphries R. K., Ley T. J., Anagnou N. P., Baur A. W., Nienhuis A. W. Beta O-39 thalassemia gene: a premature termination codon causes beta-mRNA deficiency without affecting cytoplasmic beta-mRNA stability. Blood. 1984 Jul;64(1):23–32. [PubMed] [Google Scholar]
- Karpati G., Zubrzycka-Gaarn E. E., Carpenter S., Bulman D. E., Ray P. N., Worton R. G. Age-related conversion of dystrophin-negative to -positive fiber segments of skeletal but not cardiac muscle fibers in heterozygote mdx mice. J Neuropathol Exp Neurol. 1990 Mar;49(2):96–105. doi: 10.1097/00005072-199003000-00002. [DOI] [PubMed] [Google Scholar]
- Klamut H. J., Zubrzycka-Gaarn E. E., Bulman D. E., Malhotra S. B., Bodrug S. E., Worton R. G., Ray P. N. Myogenic regulation of dystrophin gene expression. Br Med Bull. 1989 Jul;45(3):681–702. doi: 10.1093/oxfordjournals.bmb.a072352. [DOI] [PubMed] [Google Scholar]
- Knudson C. M., Hoffman E. P., Kahl S. D., Kunkel L. M., Campbell K. P. Evidence for the association of dystrophin with the transverse tubular system in skeletal muscle. J Biol Chem. 1988 Jun 15;263(17):8480–8484. [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Hoffman E. P., Bertelson C. J., Monaco A. P., Feener C., Kunkel L. M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987 Jul 31;50(3):509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malhotra S. B., Hart K. A., Klamut H. J., Thomas N. S., Bodrug S. E., Burghes A. H., Bobrow M., Harper P. S., Thompson M. W., Ray P. N. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988 Nov 4;242(4879):755–759. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- Nicholson L. V., Davison K., Johnson M. A., Slater C. R., Young C., Bhattacharya S., Gardner-Medwin D., Harris J. B. Dystrophin in skeletal muscle. II. Immunoreactivity in patients with Xp21 muscular dystrophy. J Neurol Sci. 1989 Dec;94(1-3):137–146. doi: 10.1016/0022-510x(89)90224-4. [DOI] [PubMed] [Google Scholar]
- Partridge T. A., Morgan J. E., Coulton G. R., Hoffman E. P., Kunkel L. M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989 Jan 12;337(6203):176–179. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- Patel K., Leevers S., Abbs S., Hart K. A., Heckmatt J. Z., Bobrow M., Dubowitz V. Absence of dystrophin in Becker muscular dystrophy. Lancet. 1989 Jan 7;1(8628):47–47. doi: 10.1016/s0140-6736(89)91705-4. [DOI] [PubMed] [Google Scholar]
- Patel K., Voit T., Dunn M. J., Strong P. N., Dubowitz V. Dystrophin and nebulin in the muscular dystrophies. J Neurol Sci. 1988 Nov;87(2-3):315–326. doi: 10.1016/0022-510x(88)90256-0. [DOI] [PubMed] [Google Scholar]
- Rowland L. P. Biochemistry of muscle membranes in Duchenne muscular dystrophy. Muscle Nerve. 1980 Jan-Feb;3(1):3–20. doi: 10.1002/mus.880030103. [DOI] [PubMed] [Google Scholar]
- Salviati G., Betto R., Ceoldo S., Biasia E., Bonilla E., Miranda A. F., Dimauro S. Cell fractionation studies indicate that dystrophin is a protein of surface membranes of skeletal muscle. Biochem J. 1989 Mar 15;258(3):837–841. doi: 10.1042/bj2580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicinski P., Geng Y., Ryder-Cook A. S., Barnard E. A., Darlison M. G., Barnard P. J. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989 Jun 30;244(4912):1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Takeshita K., Forget B. G., Scarpa A., Benz E. J., Jr Intranuclear defect in beta-globin mRNA accumulation due to a premature translation termination codon. Blood. 1984 Jul;64(1):13–22. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins S. C., Hoffman E. P., Slayter H. S., Kunkel L. M. Immunoelectron microscopic localization of dystrophin in myofibres. Nature. 1988 Jun 30;333(6176):863–866. doi: 10.1038/333863a0. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn E. E., Bulman D. E., Karpati G., Burghes A. H., Belfall B., Klamut H. J., Talbot J., Hodges R. S., Ray P. N., Worton R. G. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988 Jun 2;333(6172):466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- den Dunnen J. T., Bakker E., Breteler E. G., Pearson P. L., van Ommen G. J. Direct detection of more than 50% of the Duchenne muscular dystrophy mutations by field inversion gels. Nature. 1987 Oct 15;329(6140):640–642. doi: 10.1038/329640a0. [DOI] [PubMed] [Google Scholar]