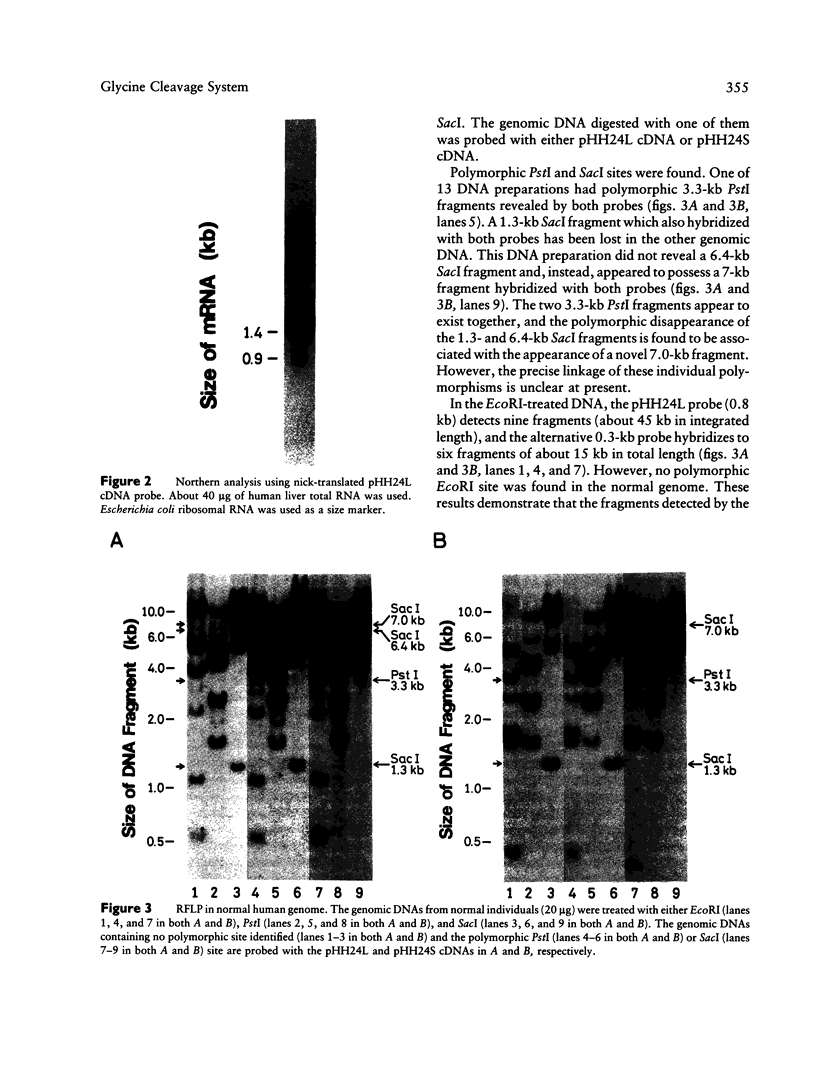
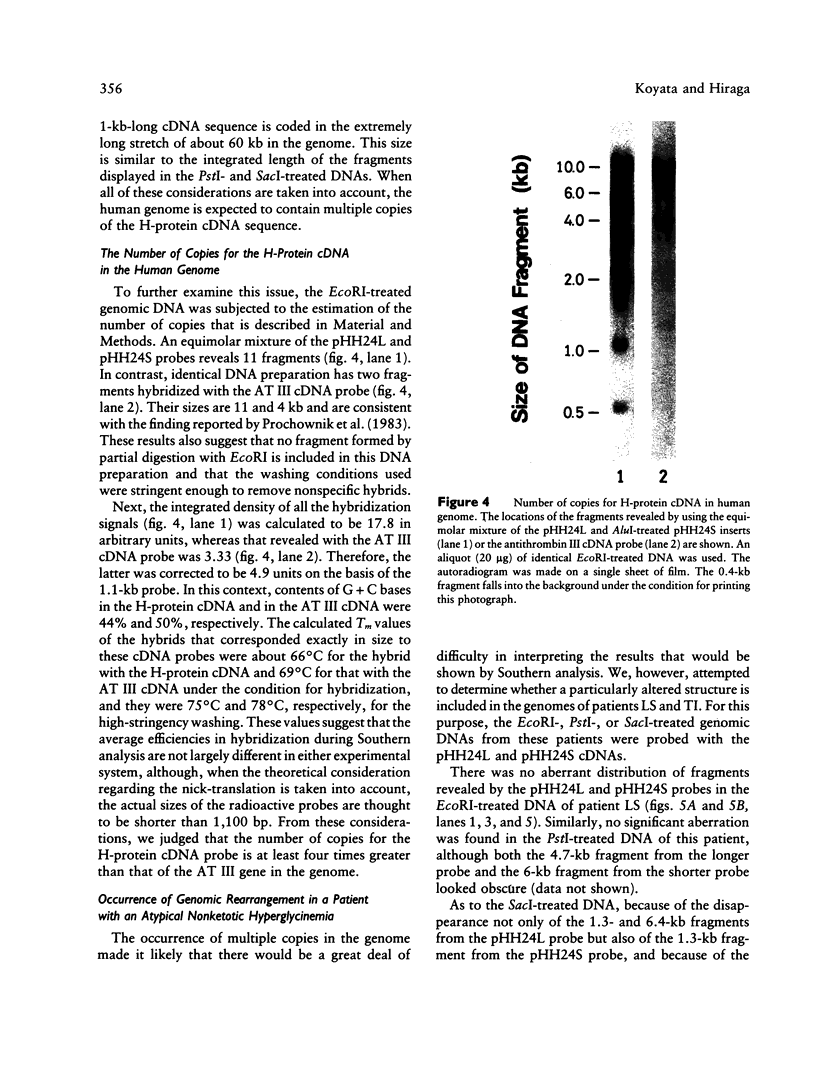
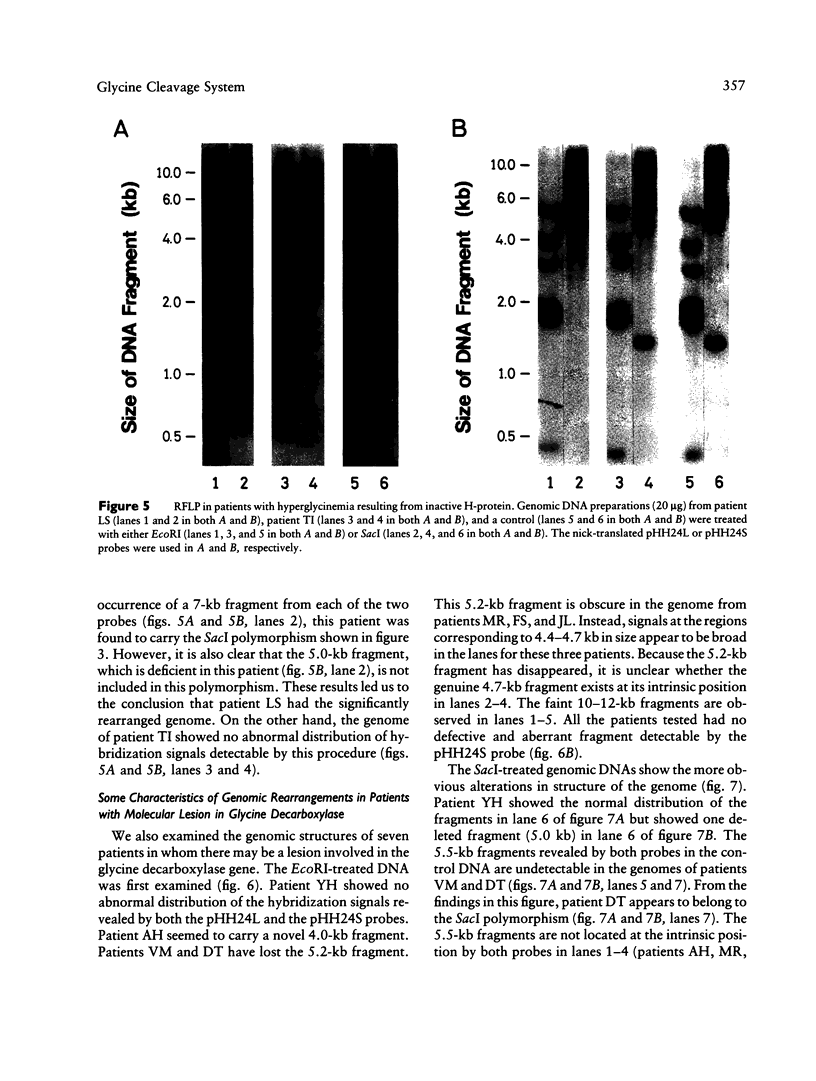
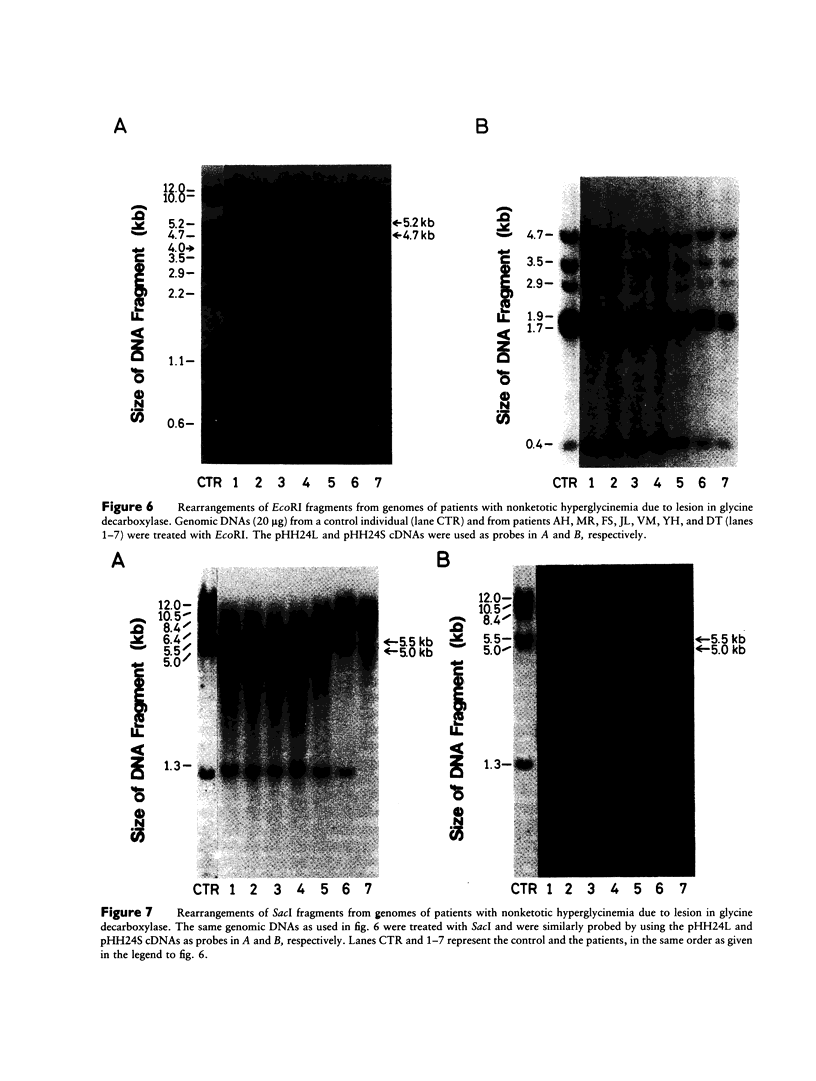
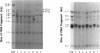Abstract
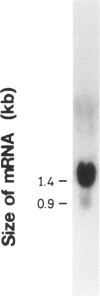
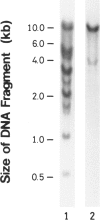
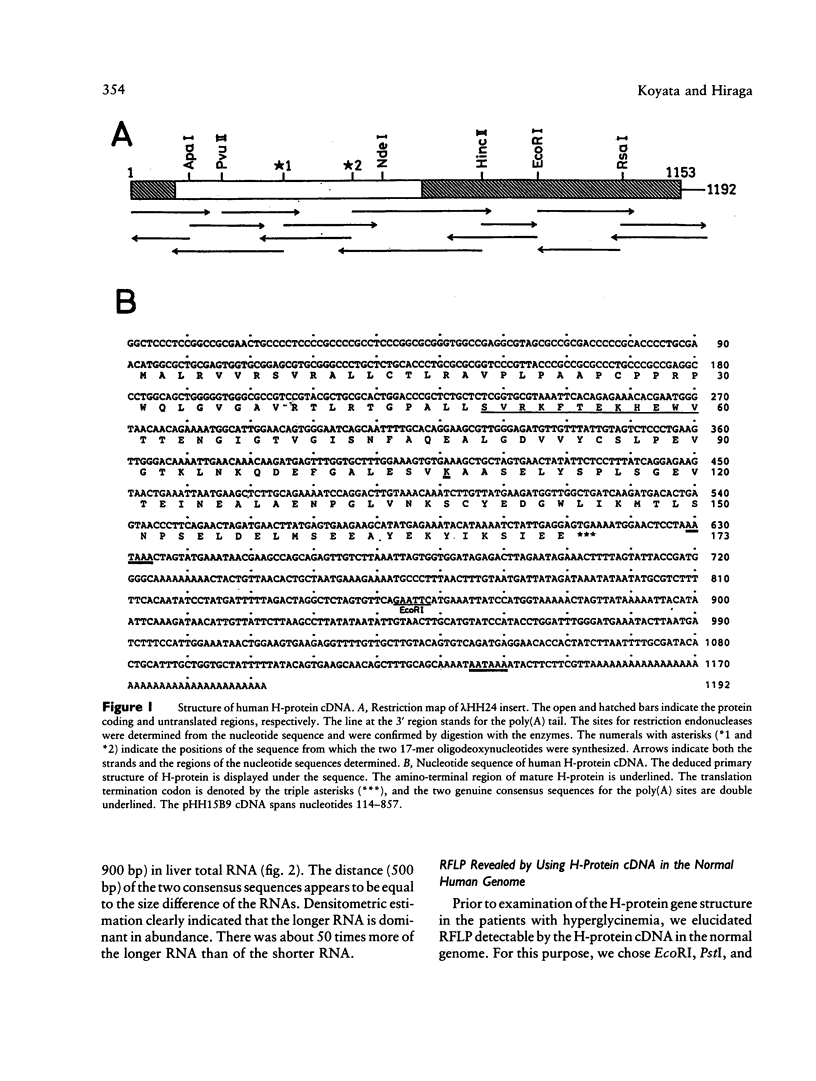
We have isolated a 1,192-base-long cDNA which encodes the entire structure of a precursor form of human H-protein. The tentatively calculated number of copies for this cDNA appeared to be about four times as many as that of the antithrombin III gene specified by a single locus in the human haploid genome. Southern analysis using H-protein cDNA probe demonstrates a deletion of the 5.0-kb SacI fragment in the genome of a patient with an atypical nonketotic hyperglycinemia in whom there was an inactive H-protein. This SacI fragment was also deleted from the genome of one of seven patients with nonketotic hyperglycinemia resulting from the lesion of glycine decarboxylase. The remaining six patients had common aberrations identified with the 5.2-kb EcoRI and 5.5-kb SacI fragments. Although implication of these defective fragments in pathogenesis is unclear at present, it is suggested that rearrangements occur in multiple genomic loci of patients with nonketotic hyperglycinemia and that this H-protein cDNA can be used for carrier screening.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner R., Ando T., Nyhan W. L. Nonketotic hyperglycinemia. J Pediatr. 1969 Dec;75(6):1022–1030. doi: 10.1016/s0022-3476(69)80341-0. [DOI] [PubMed] [Google Scholar]
- Bock S. C., Harris J. F., Balazs I., Trent J. M. Assignment of the human antithrombin III structural gene to chromosome 1q23-25. Cytogenet Cell Genet. 1985;39(1):67–69. doi: 10.1159/000132105. [DOI] [PubMed] [Google Scholar]
- Brun A., Börjeson M., Hultberg B., Sjöblad S., Akesson H., Litwin E. Neonatal non-ketotic hyperglycinemia: a clinical, biochemical and neuropathological study including electronmicroscopic findings. Neuropadiatrie. 1979 May;10(2):195–205. doi: 10.1055/s-0028-1085325. [DOI] [PubMed] [Google Scholar]
- CHILDS B., NYHAN W. L., BORDEN M., BARD L., COOKE R. E. Idiopathic hyperglycinemia and hyperglycinuria: a new disorder of amino acid metabolism. I. Pediatrics. 1961 Apr;27:522–538. [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Cloning large segments of genomic DNA using cosmid vectors. Methods Enzymol. 1987;152:199–212. doi: 10.1016/0076-6879(87)52021-3. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Okamura-Ikeda K., Motokawa Y. Chicken liver H-protein, a component of the glycine cleavage system. Amino acid sequence and identification of the N epsilon-lipoyllysine residue. J Biol Chem. 1986 Jul 5;261(19):8836–8841. [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Hayasaka K., Tada K., Fueki N., Nakamura Y., Nyhan W. L., Schmidt K., Packman S., Seashore M. R., Haan E., Danks D. M. Nonketotic hyperglycinemia: analyses of glycine cleavage system in typical and atypical cases. J Pediatr. 1987 Jun;110(6):873–877. doi: 10.1016/s0022-3476(87)80399-2. [DOI] [PubMed] [Google Scholar]
- Hayasaka K., Tada K., Kikuchi G., Winter S., Nyhan W. L. Nonketotic hyperglycinemia: two patients with primary defects of P-protein and T-protein, respectively, in the glycine cleavage system. Pediatr Res. 1983 Dec;17(12):967–970. doi: 10.1203/00006450-198312000-00008. [DOI] [PubMed] [Google Scholar]
- Hiraga K., Kikuchi G. The mitochondrial glycine cleavage system. Purification and properties of glycine decarboxylase from chicken liver mitochondria. J Biol Chem. 1980 Dec 25;255(24):11664–11670. [PubMed] [Google Scholar]
- Hiraga K., Kochi H., Hayasaka K., Kikuchi G., Nyhan W. L. Defective glycine cleavage system in nonketotic hyperglycinemia. Occurrence of a less active glycine decarboxylase and an abnormal aminomethyl carrier protein. J Clin Invest. 1981 Aug;68(2):525–534. doi: 10.1172/JCI110284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga K., Kure S., Yamamoto M., Ishiguro Y., Suzuki T. Cloning of cDNA encoding human H-protein, a constituent of the glycine cleavage system. Biochem Biophys Res Commun. 1988 Mar 15;151(2):758–762. doi: 10.1016/s0006-291x(88)80345-0. [DOI] [PubMed] [Google Scholar]
- Hollis G. F., Hieter P. A., McBride O. W., Swan D., Leder P. Processed genes: a dispersed human immunoglobulin gene bearing evidence of RNA-type processing. Nature. 1982 Mar 25;296(5855):321–325. doi: 10.1038/296321a0. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Morse H. G., Law M. L., Lidsky A., Chandra T., Woo S. L. Genetic mapping of the structural gene for antithrombin III to human chromosome 1. Hum Genet. 1984;67(1):34–36. doi: 10.1007/BF00270555. [DOI] [PubMed] [Google Scholar]
- Kikuchi G. The glycine cleavage system: composition, reaction mechanism, and physiological significance. Mol Cell Biochem. 1973 Jun 27;1(2):169–187. doi: 10.1007/BF01659328. [DOI] [PubMed] [Google Scholar]
- Kume A., Kure S., Tada K., Hiraga K. The impaired expression of glycine decarboxylase in patients with hyperglycinemias. Biochem Biophys Res Commun. 1988 Jul 15;154(1):292–297. doi: 10.1016/0006-291x(88)90683-3. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokawa Y., Hiraga K., Kochi H., Kikuchi G. Evidence for the presence of a protein-bound intermediate in the cleavage and the synthesis of glycine. Biochem Biophys Res Commun. 1970 Feb 20;38(4):771–778. doi: 10.1016/0006-291x(70)90648-0. [DOI] [PubMed] [Google Scholar]
- NYHAN W. L., BORDEN M., CHILDS B. Idiopathic hyperglycinemia: a new disorder of amino acid metabolism. II. The concentrations of other amino acids in the plasma and their modification by the administration of leucine. Pediatrics. 1961 Apr;27:539–550. [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Riaad-El Sabouty S., Dani C., Marty L., Jeanteur P. Unusual abundance of vertebrate 3-phosphate dehydrogenase pseudogenes. 1984 Nov 29-Dec 5Nature. 312(5993):469–471. doi: 10.1038/312469a0. [DOI] [PubMed] [Google Scholar]
- Prochownik E. V., Markham A. F., Orkin S. H. Isolation of a cDNA clone for human antithrombin III. J Biol Chem. 1983 Jul 10;258(13):8389–8394. [PubMed] [Google Scholar]
- Rosenberg L. E., Lilljeqvist A. C., Hsia Y. E. Methylmalonic aciduria. An inborn error leading to metabolic acidosis, long-chain ketonuria and intermittent hyperglycinemia. N Engl J Med. 1968 Jun 13;278(24):1319–1322. doi: 10.1056/NEJM196806132782404. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman R. M., Leech R. W., Scott C. R. The neuropathology of the nonketotic and ketotic hyperglycinemias: three cases. Neurology. 1978 Feb;28(2):139–146. doi: 10.1212/wnl.28.2.139. [DOI] [PubMed] [Google Scholar]
- Tada K., Hayasaka K. Non-ketotic hyperglycinaemia: clinical and biochemical aspects. Eur J Pediatr. 1987 May;146(3):221–227. doi: 10.1007/BF00716464. [DOI] [PubMed] [Google Scholar]
- Trauner D. A., Page T., Greco C., Sweetman L., Kulovich S., Nyhan W. L. Progressive neurodegenerative disorder in a patient with nonketotic hyperglycinemia. J Pediatr. 1981 Feb;98(2):272–275. doi: 10.1016/s0022-3476(81)80659-2. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Major pathways of glycine and serine catabolism in rat liver. Arch Biochem Biophys. 1970 Aug;139(2):380–392. doi: 10.1016/0003-9861(70)90490-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Kikuchi G. Physiological significance of glycine cleavage system in human liver as revealed by the study of a case of hyperglycinemia. Biochem Biophys Res Commun. 1969 May 22;35(4):577–583. doi: 10.1016/0006-291x(69)90387-8. [DOI] [PubMed] [Google Scholar]