Abstract
MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes) is a major subgroup of heterogeneous mitochondrial diseases. For identifying a mutation in the mitochondrial gene, we isolated, from the same muscle tissue from a patient with MELAS, cell lines with distinctly different phenotypes: one was respiration-deficient, and the other was apparently normal. Compared with the normal cells, only one A-to-G nucleotide transition at nucleotide 3243 in the tRNA-Leu (UUR) gene was found in whole mtDNA of the respiration-deficient cells. This mutation was also found in eight patients, from unrelated families, who had MELAS in a heteroplasmic manner but was not found in control individuals. Therefore, the single point mutation causes the functional abnormality in the respiratory chain of mitochondria.
Full text
PDF



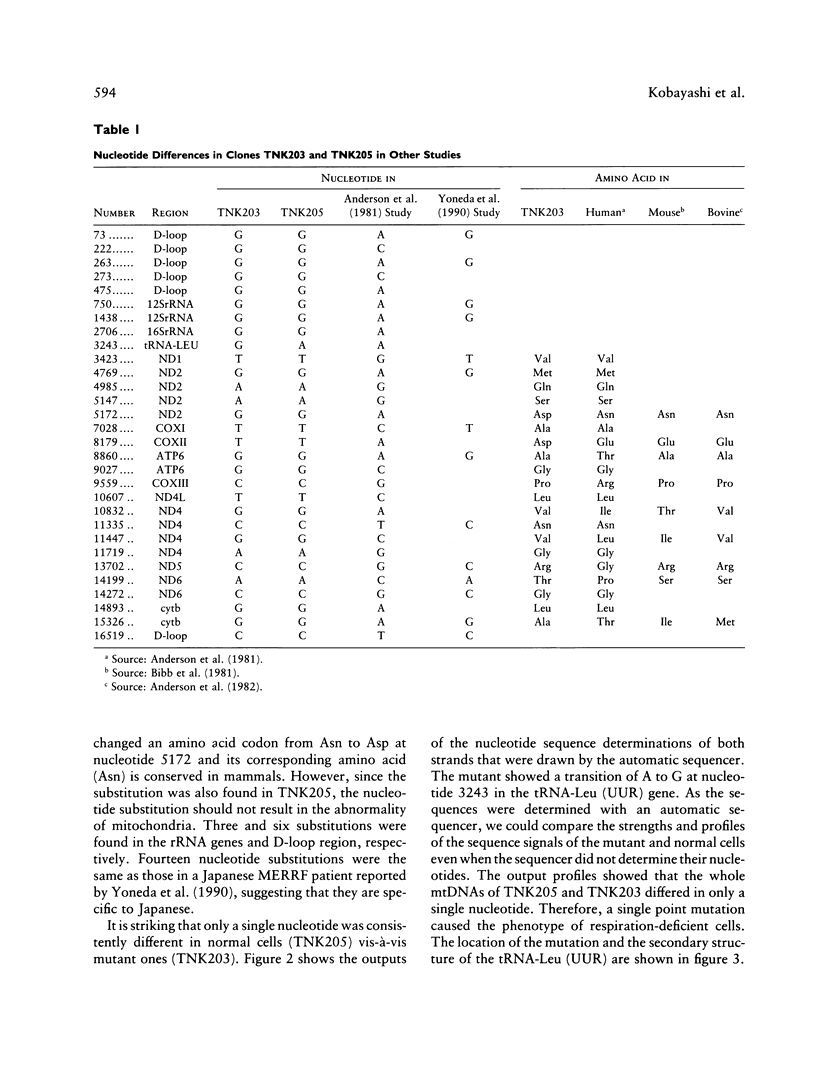
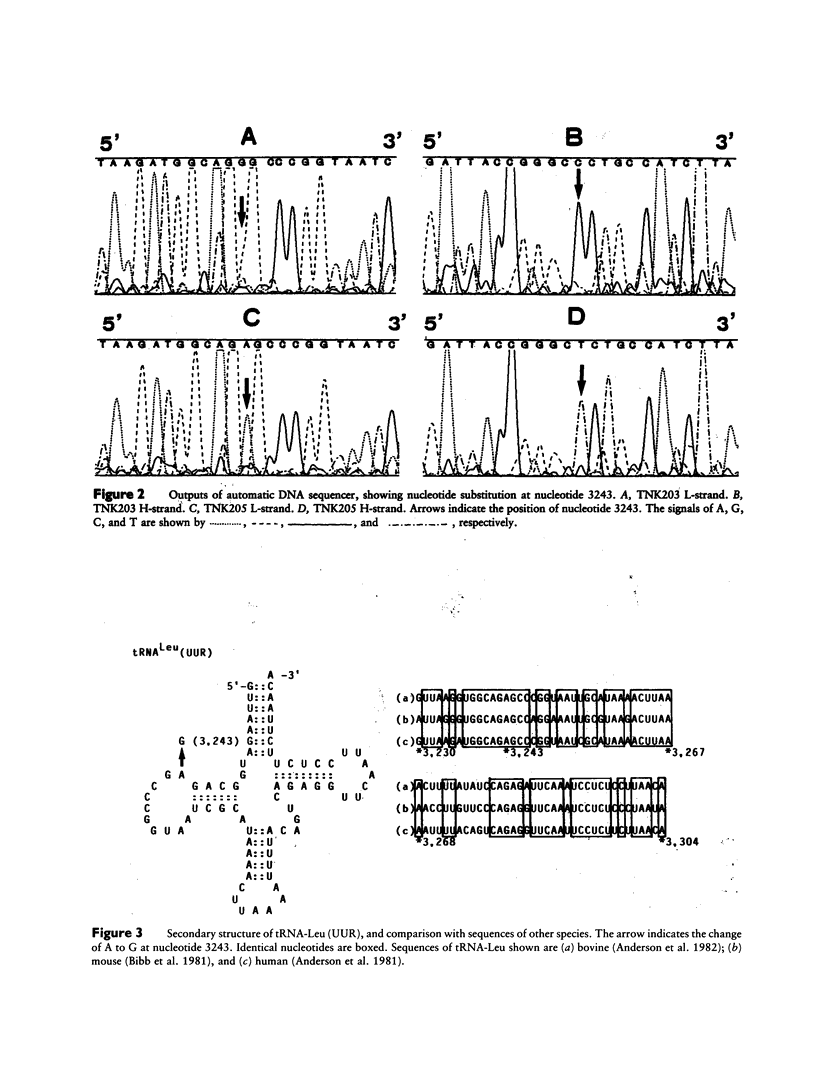
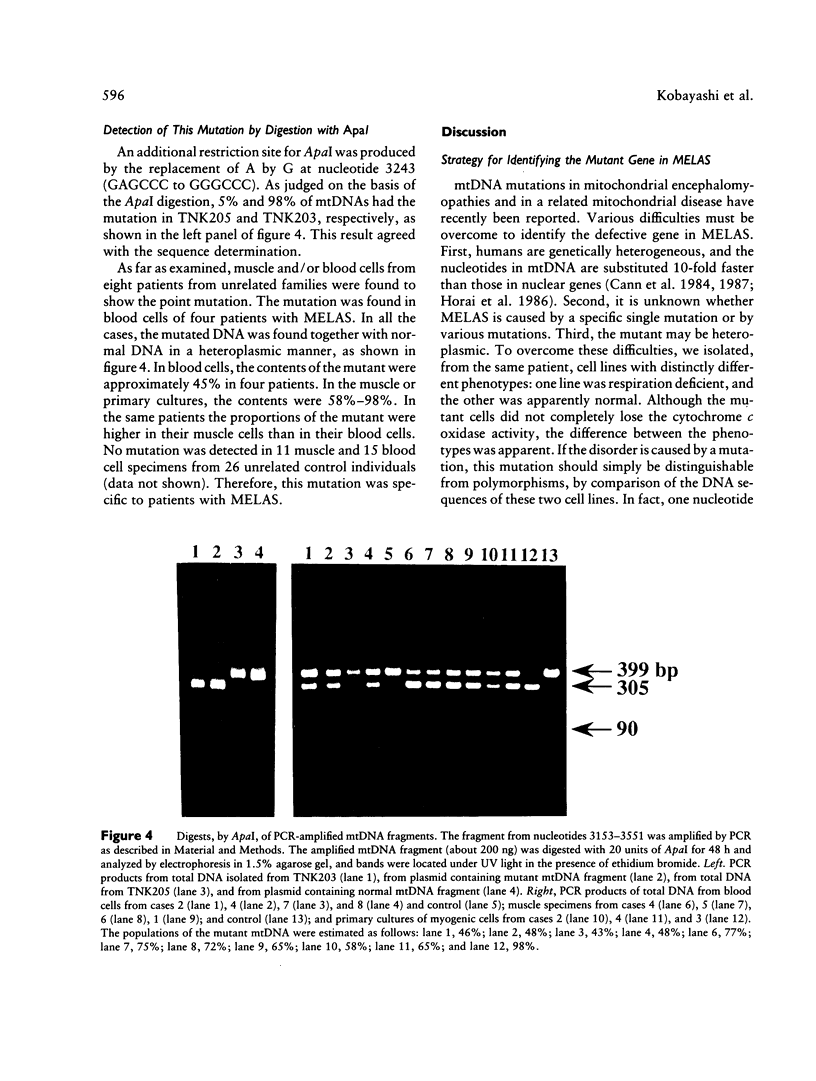



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Berenberg R. A., Pellock J. M., DiMauro S., Schotland D. L., Bonilla E., Eastwood A., Hays A., Vicale C. T., Behrens M., Chutorian A. Lumping or splitting? "Ophthalmoplegia-plus" or Kearns-Sayre syndrome? Ann Neurol. 1977 Jan;1(1):37–54. doi: 10.1002/ana.410010104. [DOI] [PubMed] [Google Scholar]
- Bibb M. J., Van Etten R. A., Wright C. T., Walberg M. W., Clayton D. A. Sequence and gene organization of mouse mitochondrial DNA. Cell. 1981 Oct;26(2 Pt 2):167–180. doi: 10.1016/0092-8674(81)90300-7. [DOI] [PubMed] [Google Scholar]
- Cann R. L., Brown W. M., Wilson A. C. Polymorphic sites and the mechanism of evolution in human mitochondrial DNA. Genetics. 1984 Mar;106(3):479–499. doi: 10.1093/genetics/106.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann R. L., Stoneking M., Wilson A. C. Mitochondrial DNA and human evolution. Nature. 1987 Jan 1;325(6099):31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- DiMauro S., Bonilla E., Zeviani M., Nakagawa M., DeVivo D. C. Mitochondrial myopathies. Ann Neurol. 1985 Jun;17(6):521–538. doi: 10.1002/ana.410170602. [DOI] [PubMed] [Google Scholar]
- ENGEL W. K., CUNNINGHAM G. G. RAPID EXAMINATION OF MUSCLE TISSUE. AN IMPROVED TRICHROME METHOD FOR FRESH-FROZEN BIOPSY SECTIONS. Neurology. 1963 Nov;13:919–923. doi: 10.1212/wnl.13.11.919. [DOI] [PubMed] [Google Scholar]
- Fukuhara N., Tokiguchi S., Shirakawa K., Tsubaki T. Myoclonus epilepsy associated with ragged-red fibres (mitochondrial abnormalities ): disease entity or a syndrome? Light-and electron-microscopic studies of two cases and review of literature. J Neurol Sci. 1980 Jul;47(1):117–133. doi: 10.1016/0022-510x(80)90031-3. [DOI] [PubMed] [Google Scholar]
- Gaines G., Rossi C., Attardi G. Markedly different ATP requirements for rRNA synthesis and mtDNA light strand transcription versus mRNA synthesis in isolated human mitochondria. J Biol Chem. 1987 Feb 5;262(4):1907–1915. [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Horai S., Matsunaga E. Mitochondrial DNA polymorphism in Japanese. II. Analysis with restriction enzymes of four or five base pair recognition. Hum Genet. 1986 Feb;72(2):105–117. doi: 10.1007/BF00283927. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Turnbull D. M., Dick D. J., Sherratt H. S. A partial deficiency of cytochrome c oxidase in chronic progressive external ophthalmoplegia. J Neurol Sci. 1983 Jul;60(1):31–53. doi: 10.1016/0022-510x(83)90125-9. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Robertus J. D., Ladner J. E., Brown R. S., Finch J. T. Conservation of the molecular structure of yeast phenylalanine transfer RNA in two crystal forms. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3711–3715. doi: 10.1073/pnas.71.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Momoi M. Y., Tominaga K., Momoi T., Nihei K., Yanagisawa M., Kagawa Y., Ohta S. A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun. 1990 Dec 31;173(3):816–822. doi: 10.1016/s0006-291x(05)80860-5. [DOI] [PubMed] [Google Scholar]
- Kruse B., Narasimhan N., Attardi G. Termination of transcription in human mitochondria: identification and purification of a DNA binding protein factor that promotes termination. Cell. 1989 Jul 28;58(2):391–397. doi: 10.1016/0092-8674(89)90853-2. [DOI] [PubMed] [Google Scholar]
- Montagna P., Gallassi R., Medori R., Govoni E., Zeviani M., Di Mauro S., Lugaresi E., Andermann F. MELAS syndrome: characteristic migrainous and epileptic features and maternal transmission. Neurology. 1988 May;38(5):751–754. doi: 10.1212/wnl.38.5.751. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Nakamigawa T., Momoi M. Y., Momoi T., Yanagisawa M. Generation of human myogenic cell lines by the transformation of primary culture with origin-defective SV40 DNA. J Neurol Sci. 1988 Feb;83(2-3):305–319. doi: 10.1016/0022-510x(88)90077-9. [DOI] [PubMed] [Google Scholar]
- Normanly J., Abelson J. tRNA identity. Annu Rev Biochem. 1989;58:1029–1049. doi: 10.1146/annurev.bi.58.070189.005121. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Pavlakis S. G., Phillips P. C., DiMauro S., De Vivo D. C., Rowland L. P. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984 Oct;16(4):481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Karnovsky M. J., Wasserkrug H. L., Hanker J. S. Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol. 1968 Jul;38(1):1–14. doi: 10.1083/jcb.38.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servidei S., Lazaro R. P., Bonilla E., Barron K. D., Zeviani M., DiMauro S. Mitochondrial encephalomyopathy and partial cytochrome c oxidase deficiency. Neurology. 1987 Jan;37(1):58–63. doi: 10.1212/wnl.37.1.58. [DOI] [PubMed] [Google Scholar]
- Shimoizumi H., Momoi M. Y., Ohta S., Kagawa Y., Momoi T., Yanagisawa M. Cytochrome c oxidase--deficient myogenic cell lines in mitochondrial myopathy. Ann Neurol. 1989 Jun;25(6):615–621. doi: 10.1002/ana.410250614. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Yoneda M., Tanno Y., Horai S., Ozawa T., Miyatake T., Tsuji S. A common mitochondrial DNA mutation in the t-RNA(Lys) of patients with myoclonus epilepsy associated with ragged-red fibers. Biochem Int. 1990 Aug;21(5):789–796. [PubMed] [Google Scholar]
- Yuzaki M., Ohkoshi N., Kanazawa I., Kagawa Y., Ohta S. Multiple deletions in mitochondrial DNA at direct repeats of non-D-loop regions in cases of familial mitochondrial myopathy. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1352–1357. doi: 10.1016/0006-291x(89)91818-4. [DOI] [PubMed] [Google Scholar]