Abstract
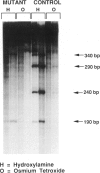
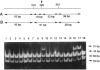
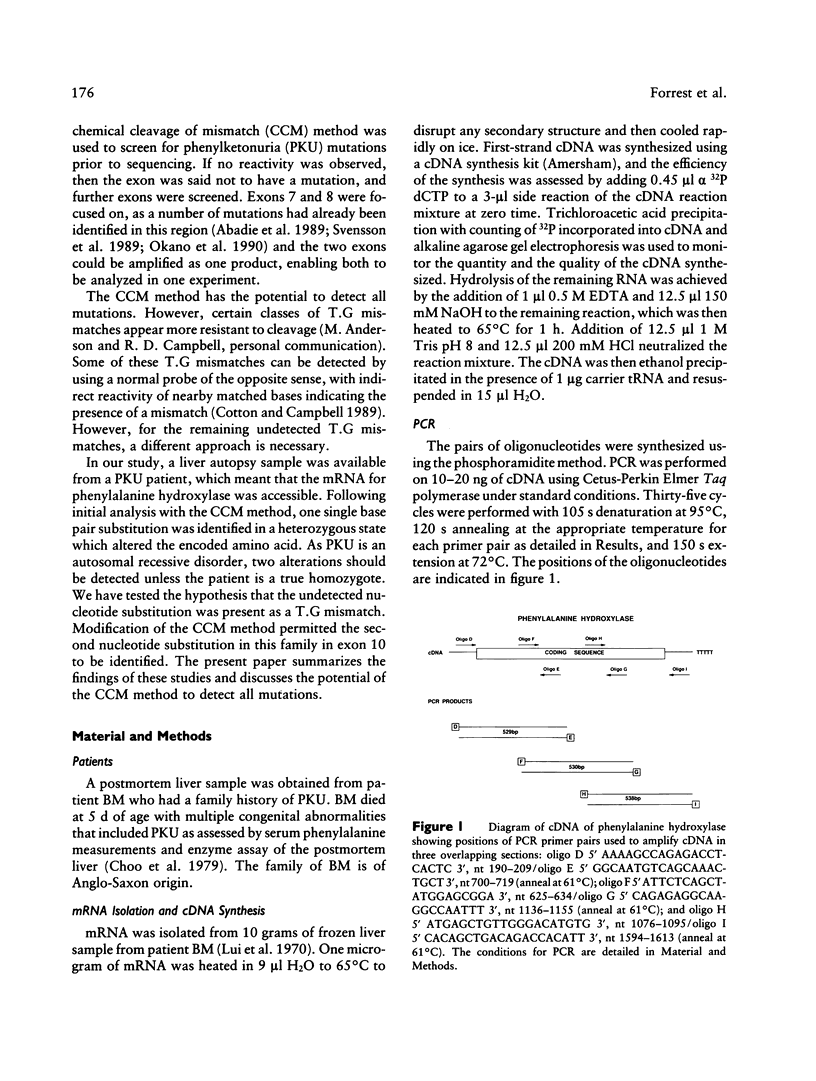
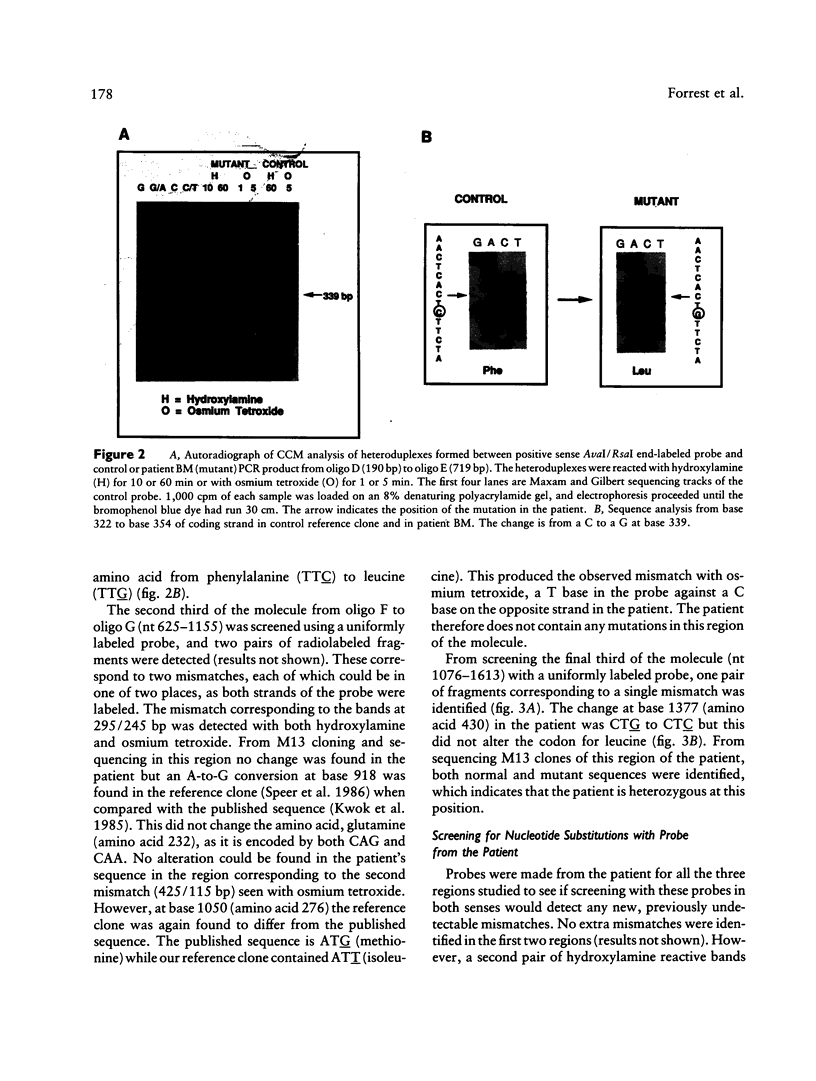
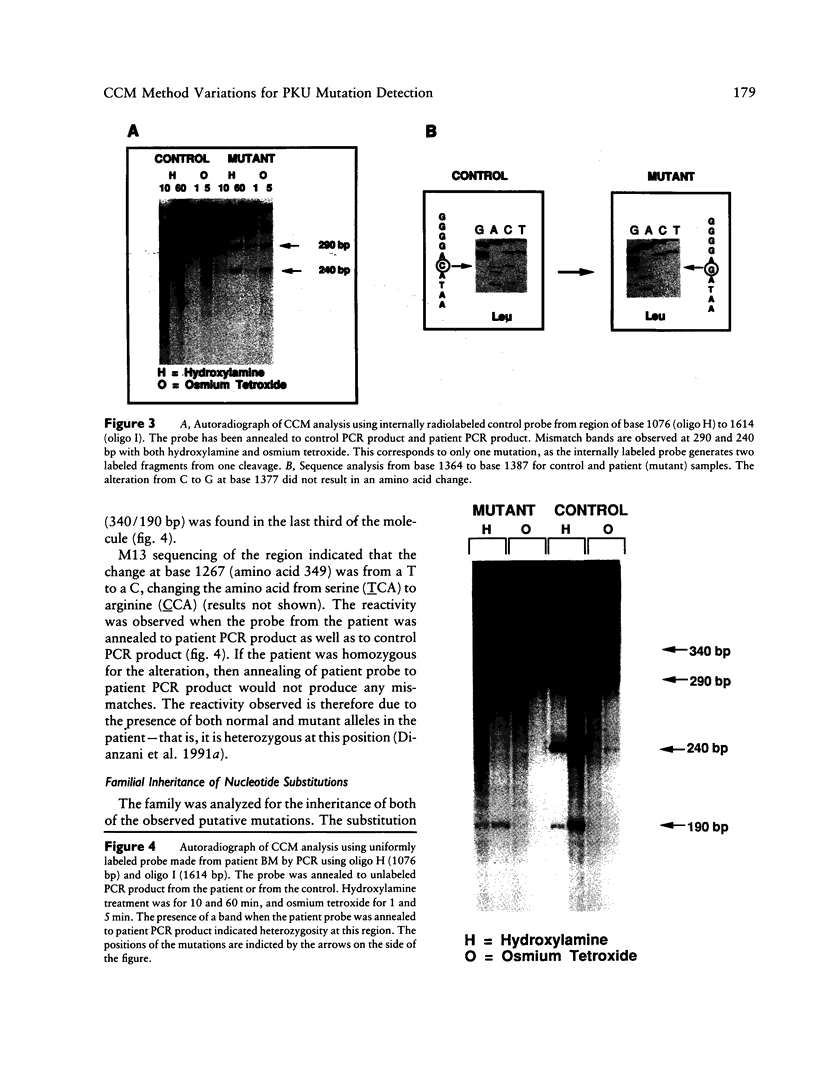
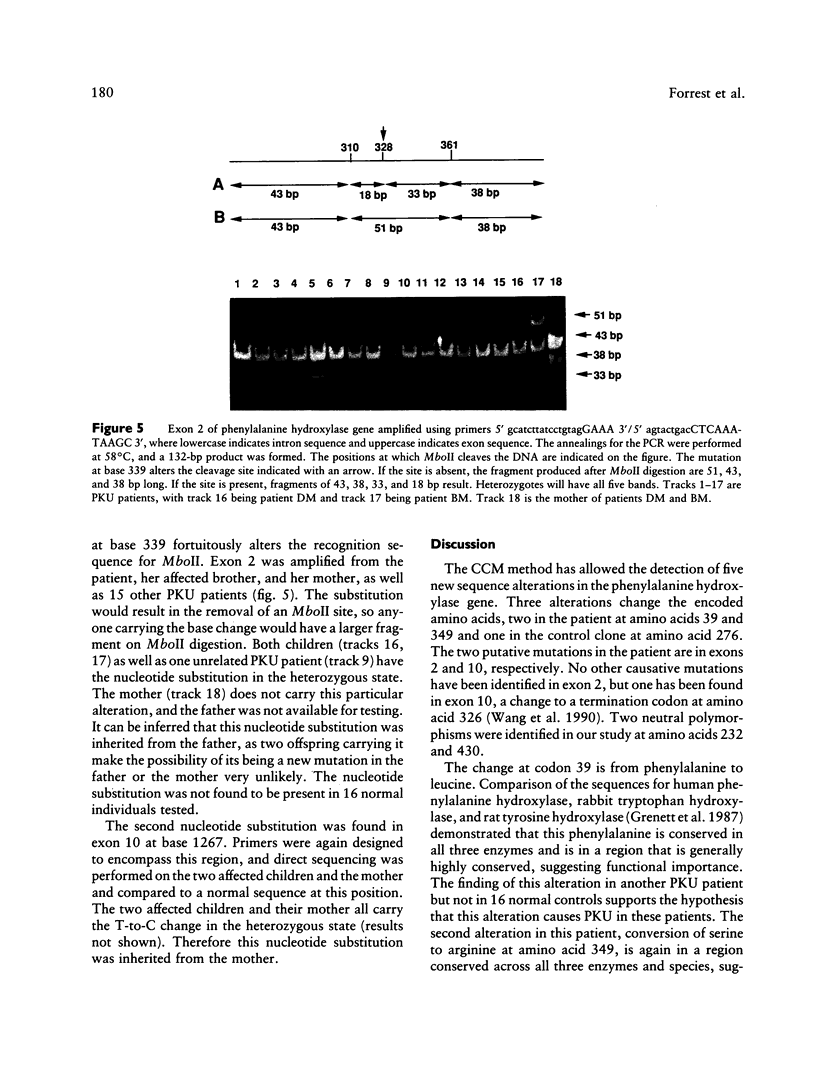
mRNA from a postmortem liver sample of a patient with classical phenylketonuria was examined using the chemical cleavage of mismatch (CCM) method to search for mutations in phenylalanine hydroxylase. Initial screening identified a heterozygous alteration in exon 2 which changed the encoded amino acid from phenylalanine (TTC) to leucine (TTG) at codon 39 and a polymorphism at codon 430 where the change from CTG to CTC did not alter the encoded leucine. Use of the CCM technique also revealed that the control reference clone differed from the published sequence by having a substitution of isoleucine (ATT) for methionine (ATG) at codon 276 and CAA rather than CAG as the codon for glutamine 232. By using the mRNA from the patient instead of the control as the source for the radiolabeled probe for the CCM technique, a second previously undetected alteration was identified in exon 10 where the change from TCA to CCA at codon 349 altered the amino acid from serine to arginine. Judicious choice of probes gives the CCM method the potential to detect close to 100% of single base mutations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadie V., Lyonnet S., Maurin N., Berthelon M., Caillaud C., Giraud F., Mattei J. F., Rey J., Rey F., Munnich A. CpG dinucleotides are mutation hot spots in phenylketonuria. Genomics. 1989 Nov;5(4):936–939. doi: 10.1016/0888-7543(89)90137-7. [DOI] [PubMed] [Google Scholar]
- Bachmann B., Lüke W., Hunsmann G. Improvement of PCR amplified DNA sequencing with the aid of detergents. Nucleic Acids Res. 1990 Mar 11;18(5):1309–1309. doi: 10.1093/nar/18.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo K. H., Cotton R. G., Danks D. M., Jennings I. G. Genetics of the mammalian phenylalanine hydroxylase system. Studies of human liver phenylalanine hydroxylase subunit structure and of mutations in phenylketonuria. Biochem J. 1979 Aug 1;181(2):285–294. doi: 10.1042/bj1810285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Campbell R. D. Chemical reactivity of matched cytosine and thymine bases near mismatched and unmatched bases in a heteroduplex between DNA strands with multiple differences. Nucleic Acids Res. 1989 Jun 12;17(11):4223–4233. doi: 10.1093/nar/17.11.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G. Detection of single base changes in nucleic acids. Biochem J. 1989 Oct 1;263(1):1–10. doi: 10.1042/bj2630001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Lamande S. R., Cotton R. G., Bateman J. F. Detection and localization of base changes in RNA using a chemical cleavage method. Anal Biochem. 1989 Dec;183(2):263–268. doi: 10.1016/0003-2697(89)90477-6. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Maragos C., Brown R. M., Hansen L. L., Brown G. K. Pyruvate dehydrogenase deficiency caused by deletion of a 7-bp repeat sequence in the E1 alpha gene. Am J Hum Genet. 1990 Aug;47(2):286–293. [PMC free article] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Brayton K., Woo S. L. An amino-acid substitution involved in phenylketonuria is in linkage disequilibrium with DNA haplotype 2. 1987 May 28-Jun 3Nature. 327(6120):333–336. doi: 10.1038/327333a0. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Dianzani I., Forrest S. M., Camaschella C., Gottardi E., Cotton R. G. Heterozygotes and homozygotes: discrimination by chemical cleavage of mismatch. Am J Hum Genet. 1991 Feb;48(2):423–424. [PMC free article] [PubMed] [Google Scholar]
- Dianzani I., Forrest S. M., Camaschella C., Saglio G., Ponzone A., Cotton R. G. Screening for mutations in the phenylalanine hydroxylase gene from Italian patients with phenylketonuria by using the chemical cleavage method: a new splice mutation. Am J Hum Genet. 1991 Mar;48(3):631–635. [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenett H. E., Ledley F. D., Reed L. L., Woo S. L. Full-length cDNA for rabbit tryptophan hydroxylase: functional domains and evolution of aromatic amino acid hydroxylases. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5530–5534. doi: 10.1073/pnas.84.16.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grompe M., Muzny D. M., Caskey C. T. Scanning detection of mutations in human ornithine transcarbamoylase by chemical mismatch cleavage. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5888–5892. doi: 10.1073/pnas.86.15.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells D. W., Forrest S. M., Dahl H. H., Cotton R. G. Insertion of an extra codon for threonine is a cause of dihydropteridine reductase deficiency. Am J Hum Genet. 1990 Aug;47(2):279–285. [PMC free article] [PubMed] [Google Scholar]
- Kwok S. C., Ledley F. D., DiLella A. G., Robson K. J., Woo S. L. Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry. 1985 Jan 29;24(3):556–561. doi: 10.1021/bi00324a002. [DOI] [PubMed] [Google Scholar]
- Liu C. P., Slate D. L., Gravel R., Ruddle F. H. Biological detection of specific mRNA molecules by microinjection. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4503–4506. doi: 10.1073/pnas.76.9.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon A. J., Green P. M., Giannelli F., Bentley D. R. Direct detection of point mutations by mismatch analysis: application to haemophilia B. Nucleic Acids Res. 1989 May 11;17(9):3347–3358. doi: 10.1093/nar/17.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Lumelsky N., Lerman L. S., Maniatis T. Detection of single base substitutions in total genomic DNA. Nature. 1985 Feb 7;313(6002):495–498. doi: 10.1038/313495a0. [DOI] [PubMed] [Google Scholar]
- Okano Y., Wang T., Eisensmith R. C., Steinmann B., Gitzelmann R., Woo S. L. Missense mutations associated with RFLP haplotypes 1 and 4 of the human phenylalanine hydroxylase gene. Am J Hum Genet. 1990 Jan;46(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- Pilz R. B., Steglich C., Scheffler I. E. Molecular and genetic characterization of an ornithine decarboxylase-deficient Chinese hamster cell line. J Biol Chem. 1990 May 25;265(15):8880–8886. [PubMed] [Google Scholar]
- Reiss J., Krawczak M., Schloesser M., Wagner M., Cooper D. N. The effect of replication errors on the mismatch analysis of PCR-amplified DNA. Nucleic Acids Res. 1990 Feb 25;18(4):973–978. doi: 10.1093/nar/18.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer A., Dahl H. H., Riess O., Cobet G., Hanke R., Cotton R. G., Coutelle C. Typing of families with classical phenylketonuria using three alleles of the Hindiii linked restriction fragment polymorphism, detectable with a phenylalanine hydroxylase cDNA probe. Family typing for PKU by linked HindIII RFLP. Clin Genet. 1986 Jun;29(6):491–495. [PubMed] [Google Scholar]