Abstract
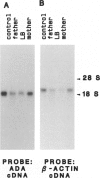
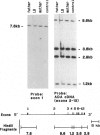
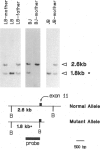
In 15%-20% of children with severe combined immunodeficiency (SCID), the underlying defect is adenosine deaminase (ADA) deficiency. The overall goal of our research has been to identify the precise molecular defects in patients with ADA-deficient SCID. In this study, we focused on a patient whom we found to have normal sized ADA mRNA by Northern analysis and an intact ADA structural gene by Southern analysis. By cloning and sequencing this patient's ADA cDNA, we found a C-to-T point mutation in exon 11. This resulted in the amino acid substitution of a valine for an alanine at position 329 of the ADA protein. Sequence analysis revealed that this mutation created a new BalI restriction site. Using Southern analyses, we were able to directly screen individuals to determine the frequency of this mutation. By combining data on eight families followed at our institution with data on five other families reported in the literature, we established that five of 13 patients (seven of 22 alleles) with known or suspected point mutations have this defect. This mutation was found to be associated with three different ADA haplotypes. This argues against a founder effect and suggests that the mutation is very old. In summary, a conservative amino acid substitution is found in a high proportion of patients with ADA deficiency; this can easily be detected by Southern analysis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian G. S., Wiginton D. A., Hutton J. J. Structure of adenosine deaminase mRNAs from normal and adenosine deaminase-deficient human cell lines. Mol Cell Biol. 1984 Sep;4(9):1712–1717. doi: 10.1128/mcb.4.9.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akeson A. L., Wiginton D. A., Dusing M. R., States J. C., Hutton J. J. Mutant human adenosine deaminase alleles and their expression by transfection into fibroblasts. J Biol Chem. 1988 Nov 5;263(31):16291–16296. [PubMed] [Google Scholar]
- Akeson A. L., Wiginton D. A., States J. C., Perme C. M., Dusing M. R., Hutton J. J. Mutations in the human adenosine deaminase gene that affect protein structure and RNA splicing. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5947–5951. doi: 10.1073/pnas.84.16.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berkvens T. M., Gerritsen E. J., Oldenburg M., Breukel C., Wijnen J. T., van Ormondt H., Vossen J. M., van der Eb A. J., Meera Khan P. Severe combined immune deficiency due to a homozygous 3.2-kb deletion spanning the promoter and first exon of the adenosine deaminase gene. Nucleic Acids Res. 1987 Nov 25;15(22):9365–9378. doi: 10.1093/nar/15.22.9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthron D. T., Markham A. F., Ginsburg D., Orkin S. H. Identification of a point mutation in the adenosine deaminase gene responsible for immunodeficiency. J Clin Invest. 1985 Aug;76(2):894–897. doi: 10.1172/JCI112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortin M. M., Rimm A. A. Severe combined immunodeficiency disease. Characterization of the disease and results of transplantation. JAMA. 1977 Aug 15;238(7):591–600. [PubMed] [Google Scholar]
- Buckley R. H., Schiff S. E., Sampson H. A., Schiff R. I., Markert M. L., Knutsen A. P., Hershfield M. S., Huang A. T., Mickey G. H., Ward F. E. Development of immunity in human severe primary T cell deficiency following haploidentical bone marrow stem cell transplantation. J Immunol. 1986 Apr 1;136(7):2398–2407. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H., Farabaugh P. J., Gilbert W. Molecular basis of base substitution hotspots in Escherichia coli. Nature. 1978 Aug 24;274(5673):775–780. doi: 10.1038/274775a0. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Brayton K., Woo S. L. An amino-acid substitution involved in phenylketonuria is in linkage disequilibrium with DNA haplotype 2. 1987 May 28-Jun 3Nature. 327(6120):333–336. doi: 10.1038/327333a0. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Lidsky A. S., Güttler F., Woo S. L. Tight linkage between a splicing mutation and a specific DNA haplotype in phenylketonuria. 1986 Aug 28-Sep 3Nature. 322(6082):799–803. doi: 10.1038/322799a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson S., Proper J. A., Bowie E. J., Sommer S. S. Parameters affecting the yield of DNA from human blood. Anal Biochem. 1987 Sep;165(2):294–299. doi: 10.1016/0003-2697(87)90272-7. [DOI] [PubMed] [Google Scholar]
- Katsuki T., Hinuma Y. Characteristics of cell lines derived from human leukocytes transformed by different strains of Epstein-Barr virus. Int J Cancer. 1975 Feb 15;15(2):203–210. doi: 10.1002/ijc.2910150205. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Schiff R. I., Buckley R. H. Adenosine deaminase and purine nucleoside phosphorylase deficiencies: evaluation of therapeutic interventions in eight patients. J Clin Immunol. 1987 Sep;7(5):389–399. doi: 10.1007/BF00917017. [DOI] [PubMed] [Google Scholar]
- Markert M. L., Hershfield M. S., Wiginton D. A., States J. C., Ward F. E., Bigner S. H., Buckley R. H., Kaufman R. E., Hutton J. J. Identification of a deletion in the adenosine deaminase gene in a child with severe combined immunodeficiency. J Immunol. 1987 May 15;138(10):3203–3206. [PubMed] [Google Scholar]
- Markert M. L., Hutton J. J., Wiginton D. A., States J. C., Kaufman R. E. Adenosine deaminase (ADA) deficiency due to deletion of the ADA gene promoter and first exon by homologous recombination between two Alu elements. J Clin Invest. 1988 May;81(5):1323–1327. doi: 10.1172/JCI113458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Daddona P. E., Shewach D. S., Markham A. F., Bruns G. A., Goff S. C., Kelley W. N. Molecular cloning of human adenosine deaminase gene sequences. J Biol Chem. 1983 Nov 10;258(21):12753–12756. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Valerio D., Dekker B. M., Duyvesteyn M. G., van der Voorn L., Berkvens T. M., van Ormondt H., van der Eb A. J. One adenosine deaminase allele in a patient with severe combined immunodeficiency contains a point mutation abolishing enzyme activity. EMBO J. 1986 Jan;5(1):113–119. doi: 10.1002/j.1460-2075.1986.tb04184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Meera Khan P., Geurts van Kessel A., de Waard A., van der Eb A. J. Isolation of cDNA clones for human adenosine deaminase. Gene. 1983 Nov;25(2-3):231–240. doi: 10.1016/0378-1119(83)90227-5. [DOI] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Friedman R. L., Suttle D. P., Hutton J. J. Cloning of cDNA sequences of human adenosine deaminase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7481–7485. doi: 10.1073/pnas.80.24.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Hutton J. J. Immunoreactive protein in adenosine deaminase deficient human lymphoblast cell lines. J Biol Chem. 1982 Mar 25;257(6):3211–3217. [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]