Abstract
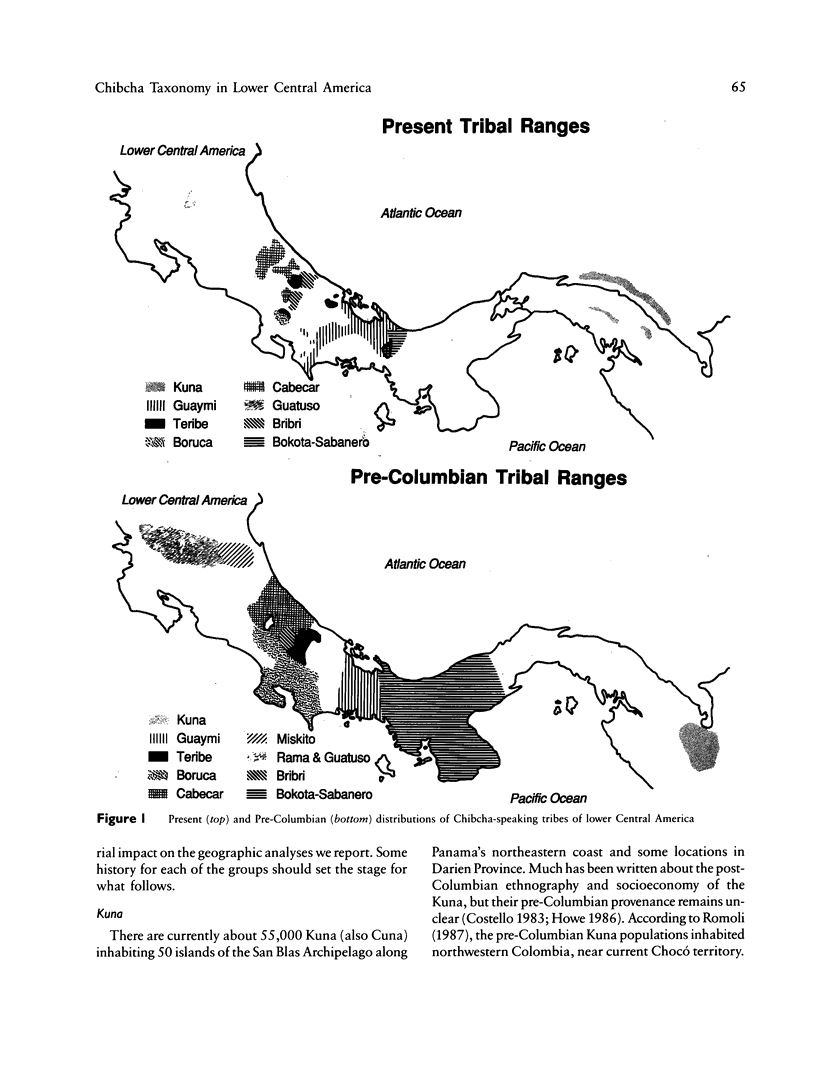
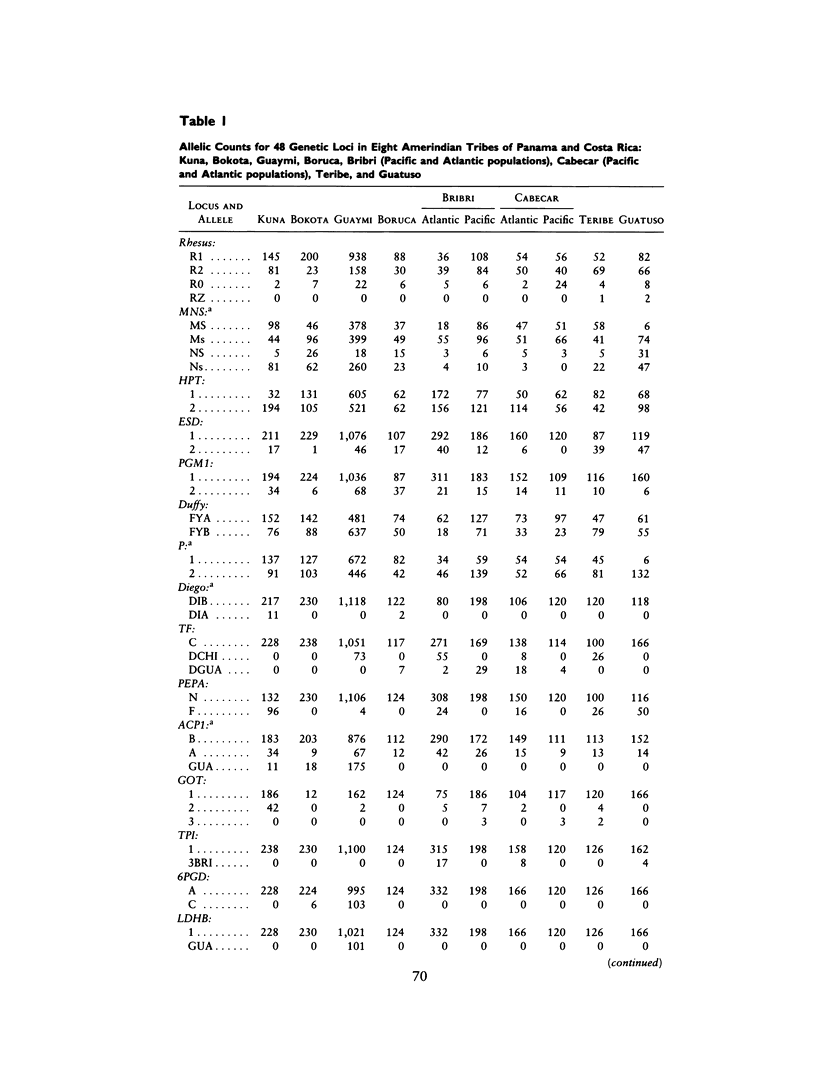
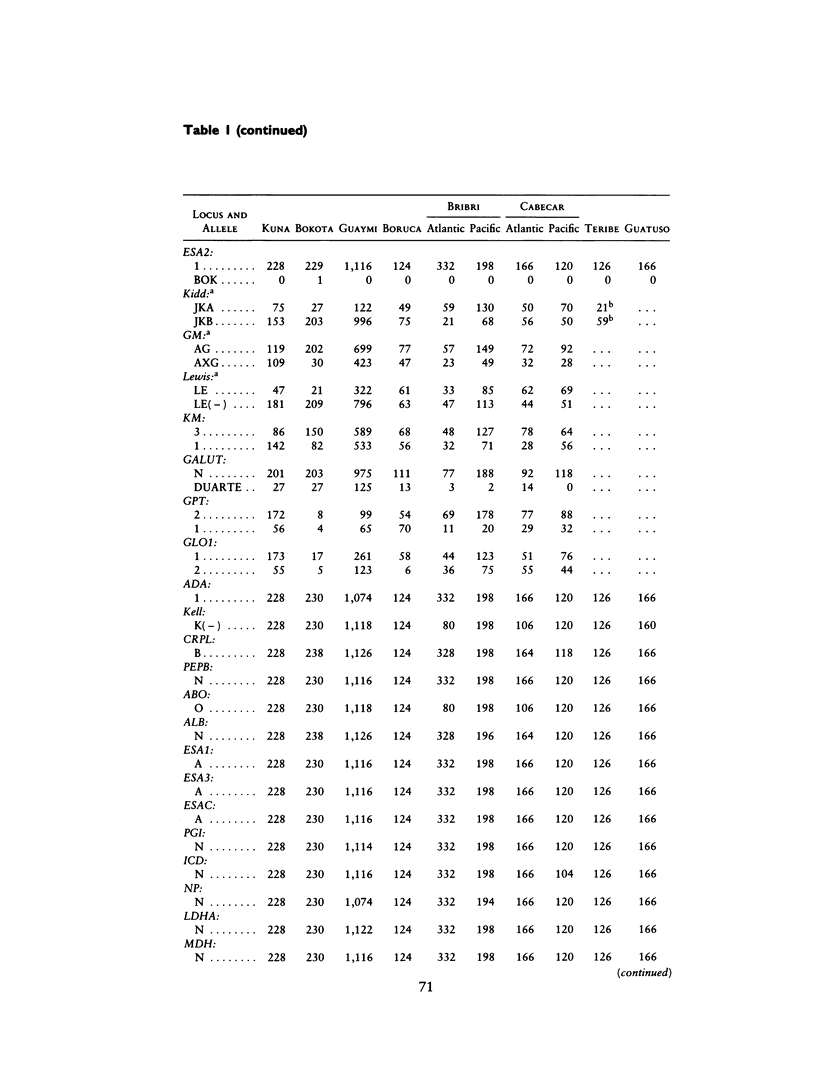
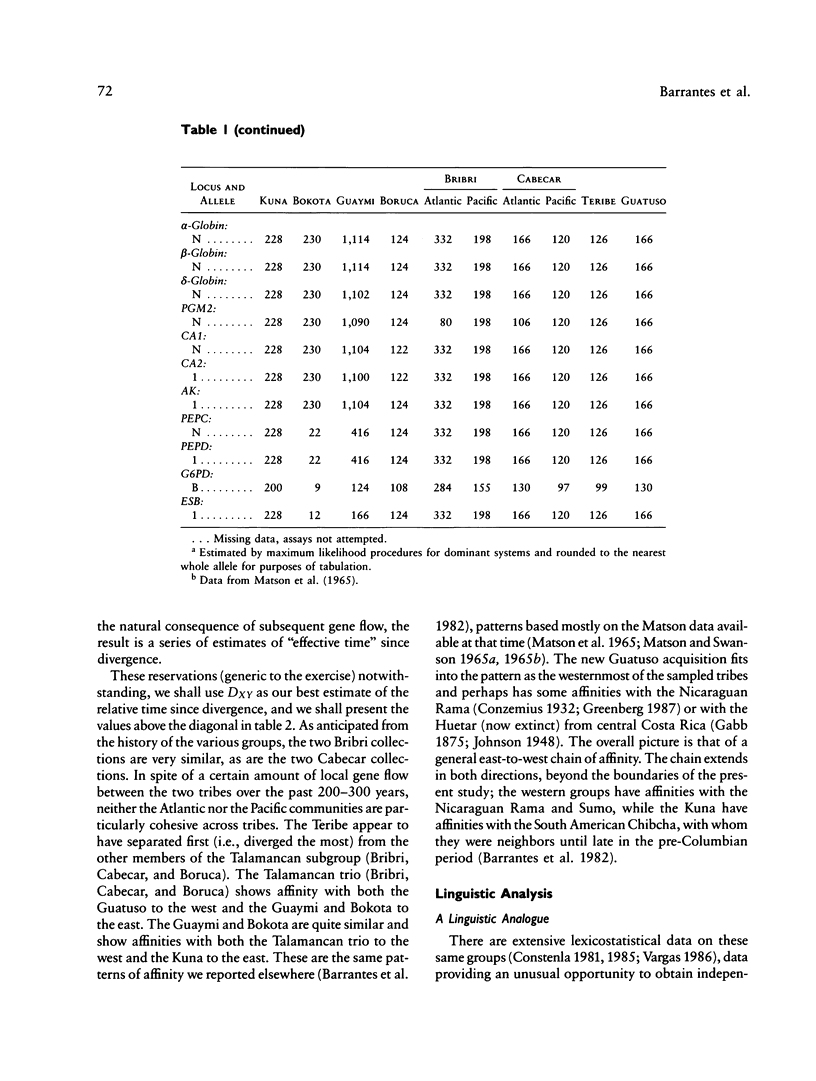
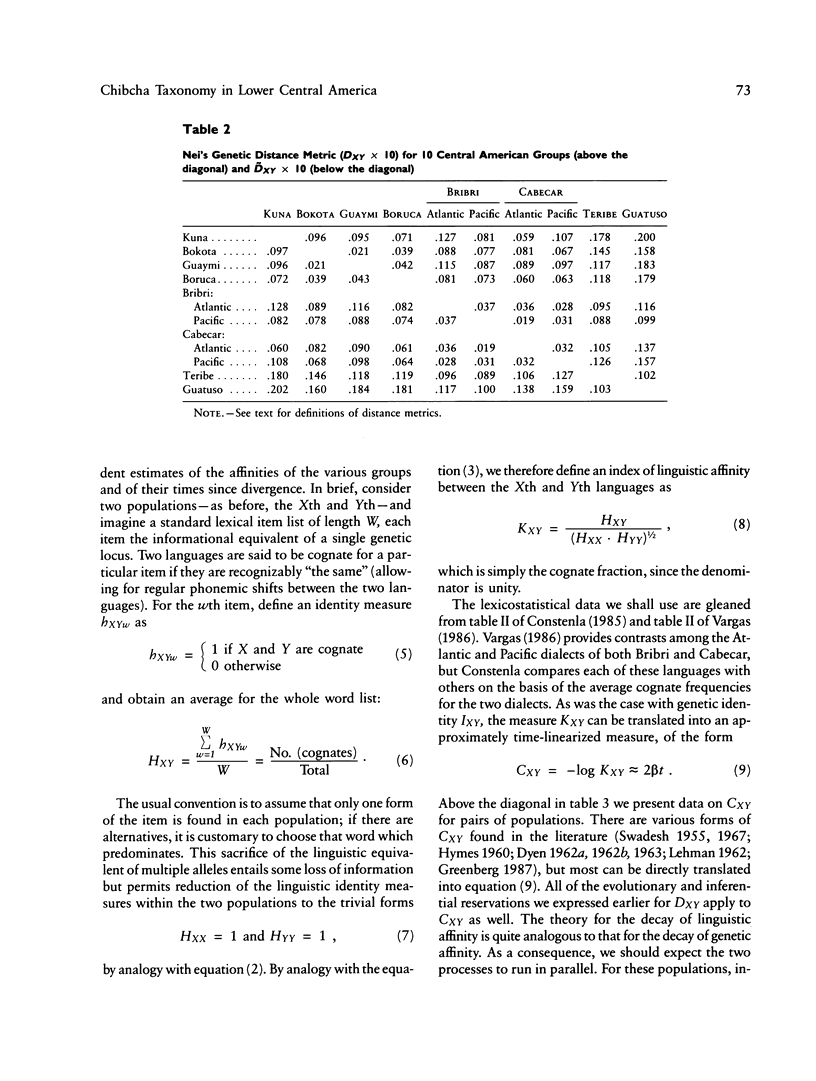
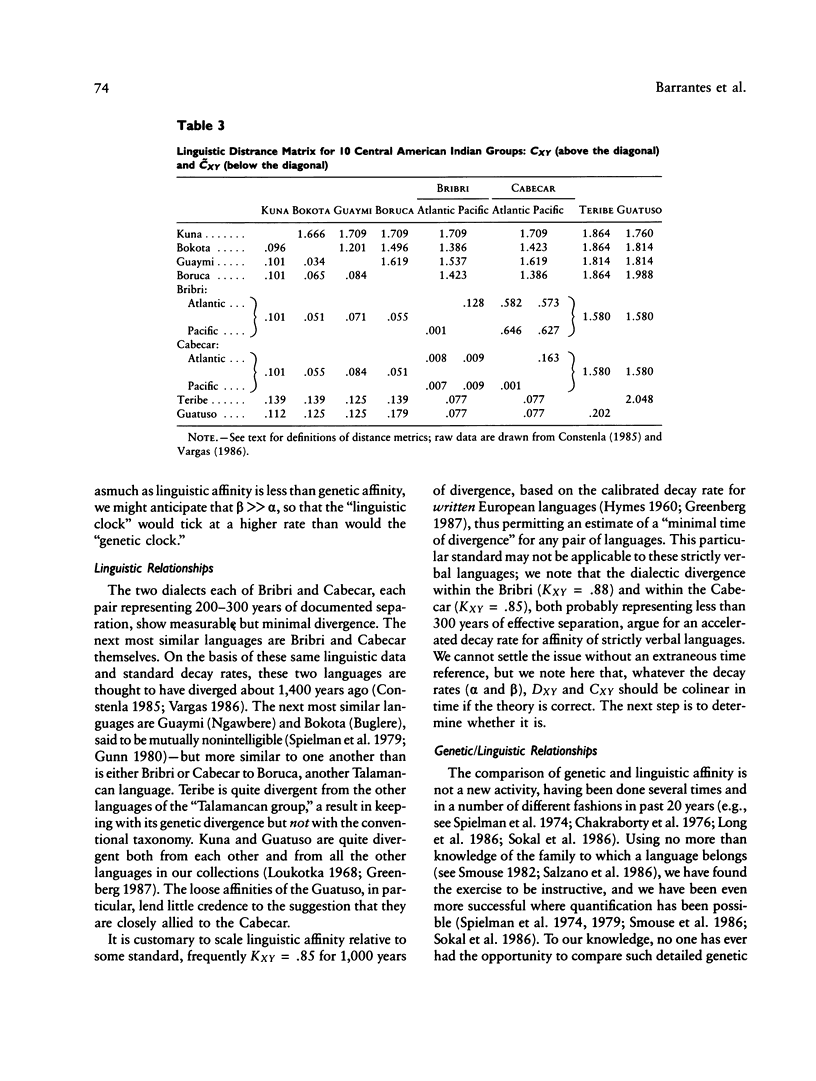
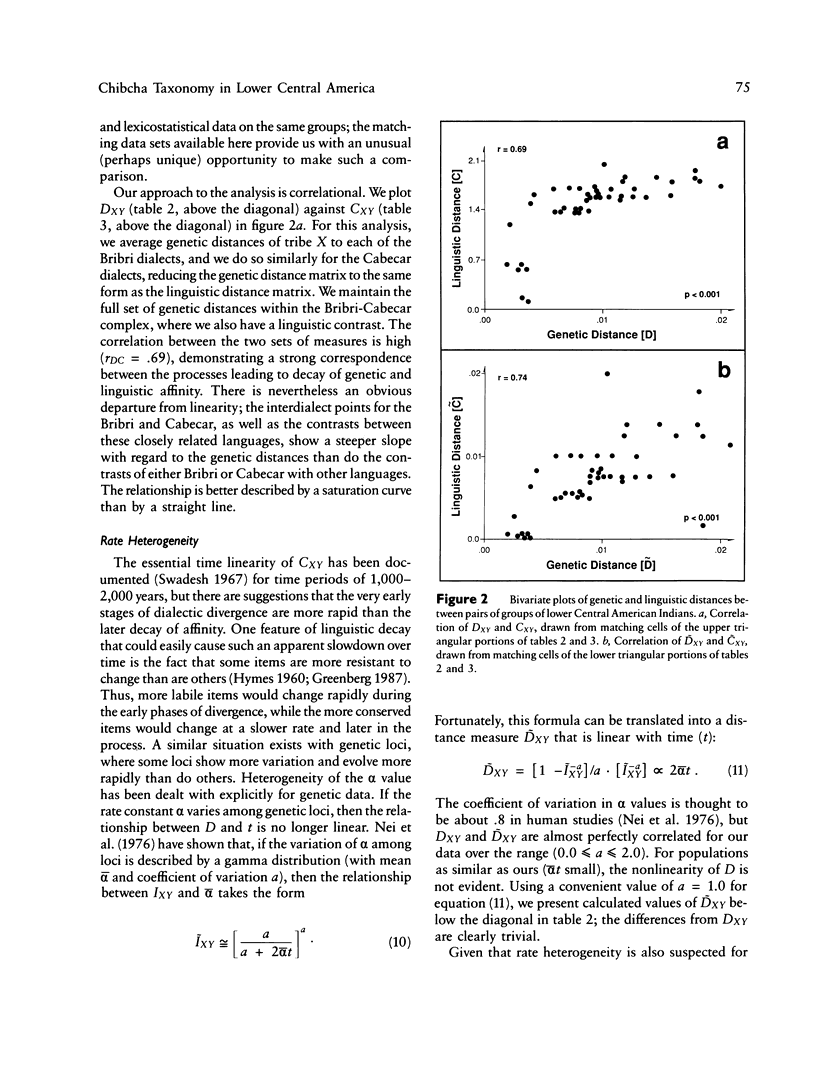
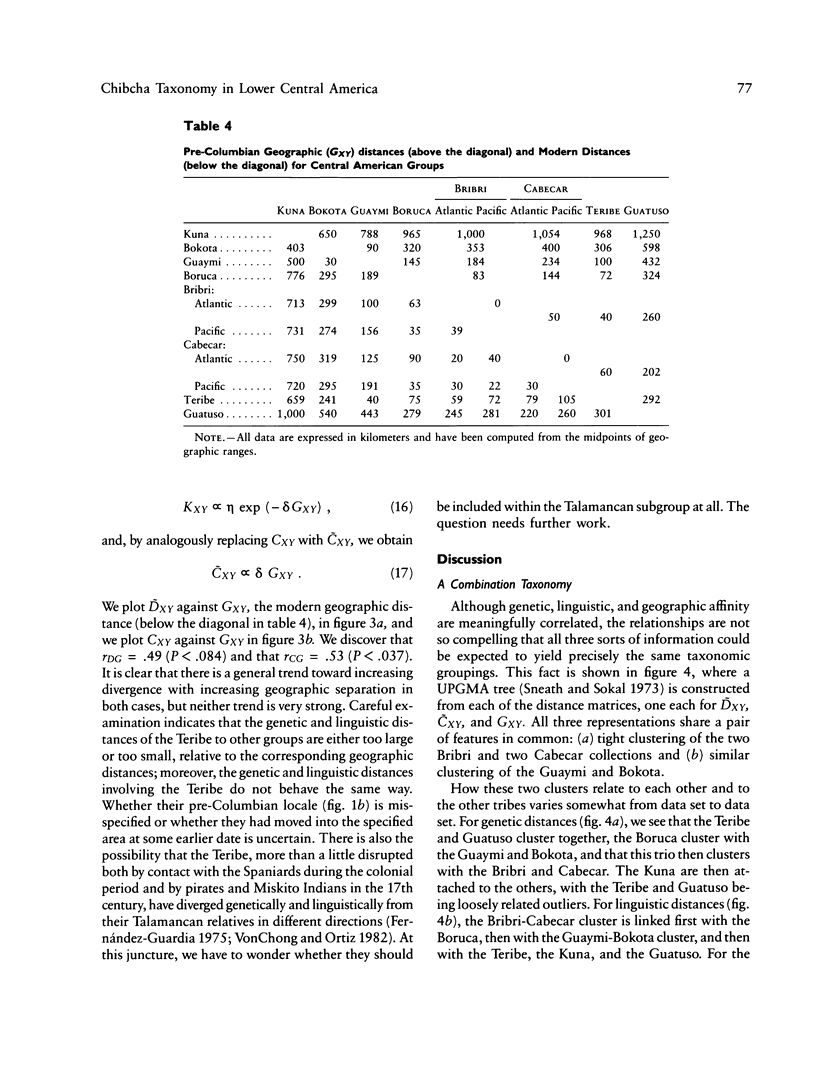
There is evidence that Amerindians have continuously occupied the lower Central American Isthmus for as long as 10,000 years. There remains some doubt about the relationships of these original colonizers to the resident peoples of this zone at the time of European contact (approximately A.D. 1500). We present new genetic data for up to 48 genetic loci for 570 members of six Chibcha-speaking tribes of lower Central America--the Boruca, Bribri, Cabecar, and Guatuso of Costa Rica and the Kuna and Teribe of Panama--and delineate the genetic affinities among the various groups (these six tribes and the Guaymi and Bokota) of lower Central America. We convert standard genetic distance metrics into a form that is linear with the effective time since divergence, and we compare the genetic distances with linguistic distances for the same groups (r = .74, P less than .001). Geographic affinity accounts for some of the genetic divergence among groups (r = .49, P less than .084) and for some of the linguistic divergence (r = .53, P less than .037), but the correspondence between geographic position and taxonomic affinity is not high. We combine all of the genetic and linguistic data to construct a synthetic overview taxonomy of the lower Central American Chibcha. Both the genetic and linguistic data exhibit hierarchical organization of tribal groups, showing a general east-to-west pattern of grouping, with greater affinities between close neighbors. The presence of private genetic variants of some antiquity within the region and their absence outside the zone, coupled with the essential absence of the DI*A polymorphism of mongoloid origin that is widespread outside the zone, argue for a relatively isolated development of the Central American Chibcha. Our results do not support the old view of lower Central America as a frontier between more advanced cultures to the north and south. Any such explanation would require recent waves of migration from outside the region, migration that is not compatible with either the genetic or linguistic data or with the archaeological history of the region.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias T. D., Inaba T., Cooke R. G., Jorge L. F. A preliminary note on the transient polymorphic oxidation of sparteine in the Ngawbé Guaymí Amerindians: a case of genetic divergence with tentative phylogenetic time frame for the pathway. Clin Pharmacol Ther. 1988 Sep;44(3):343–352. doi: 10.1038/clpt.1988.160. [DOI] [PubMed] [Google Scholar]
- Arias T. D., Jorge L. F., Lee D., Barrantes R., Inaba T. The oxidative metabolism of sparteine in the Cuna Amerindians of Panama: absence of evidence for deficient metabolizers. Clin Pharmacol Ther. 1988 Apr;43(4):456–465. doi: 10.1038/clpt.1988.58. [DOI] [PubMed] [Google Scholar]
- Barrantes R., Smouse P. E., Neel J. V., Mohrenweiser H. W., Gershowitz H. Migration and genetic infrastructure of the Central American Guaymi and their affinities with other tribal groups. Am J Phys Anthropol. 1982 Jun;58(2):201–214. doi: 10.1002/ajpa.1330580213. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Blanco R., Rothhammer F., Llop E. Genetic variability in Chilean Indian populations and its association with geography, language, and culture. Soc Biol. 1976 Spring;23(1):73–81. doi: 10.1080/19485565.1976.9988205. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Maximum-likelihood estimation of evolutionary trees from continuous characters. Am J Hum Genet. 1973 Sep;25(5):471–492. [PMC free article] [PubMed] [Google Scholar]
- Gershowitz H., Layrisse M., Layrisse Z., Neel J. V., Chagnon N., Ayres M. The genetic structure of a tirbal population, the Vanomama Indians. II. Eleven blood-group systems and the ABH-Le secretor traits. Ann Hum Genet. 1972 Mar;35(3):261–269. doi: 10.1111/j.1469-1809.1957.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Gershowitz H., Neel J. V. The immunoglobulin allotypes (Gm and Km) of twelve Indian tribes of Central and South America. Am J Phys Anthropol. 1978 Sep;49(3):289–301. doi: 10.1002/ajpa.1330490302. [DOI] [PubMed] [Google Scholar]
- Inaba T., Arias T. D. On phenotyping with isoniazid: the use of urinary acetylation ratio and the uniqueness of antimodes. Study of two Amerindian populations. Clin Pharmacol Ther. 1987 Nov;42(5):493–497. doi: 10.1038/clpt.1987.186. [DOI] [PubMed] [Google Scholar]
- Inaba T., Jorge L. F., Arias T. D. Mephenytoin hydroxylation in the Cuna Amerindians of Panama. Br J Clin Pharmacol. 1988 Jan;25(1):75–79. doi: 10.1111/j.1365-2125.1988.tb03284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R. The first Americans are getting younger. Science. 1987 Nov 27;238(4831):1230–1232. doi: 10.1126/science.3685973. [DOI] [PubMed] [Google Scholar]
- Long J. C., Smouse P. E., Wood J. W. The allelic correlation structure of Gainj- and Kalam-speaking people. II. The genetic distance between population subdivisions. Genetics. 1987 Oct;117(2):273–283. doi: 10.1093/genetics/117.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantel N. The detection of disease clustering and a generalized regression approach. Cancer Res. 1967 Feb;27(2):209–220. [PubMed] [Google Scholar]
- Matson G. A., Sutton H. E., Swanson J., Robinson A. R. Distribution of haptoglobin, transferrin, and hemoglobin types among Indians of Middle America: in British Honduras, Costa Rica and Panama. Am J Phys Anthropol. 1965 Jun;23(2):123–129. doi: 10.1002/ajpa.1330230211. [DOI] [PubMed] [Google Scholar]
- Matson G. A., Swanson J. Distribution of hereditary blood antigens among Indians in Middle America. VII. In Costa Rica. Am J Phys Anthropol. 1965 Jun;23(2):107–121. doi: 10.1002/ajpa.1330230210. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Allen R. C. Useful buffer and gel systems for polyacrylamide gel electrophoresis. Z Klin Chem Klin Biochem. 1972 May;10(5):220–225. doi: 10.1515/cclm.1972.10.5.220. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Novotny J. E. An enzymatically inactive variant of human lactate dehydrogenase-LDHBGUA-1. Study of subunit interaction. Biochim Biophys Acta. 1982 Mar 18;702(1):90–98. doi: 10.1016/0167-4838(82)90030-9. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Wurzinger K. H., Neel J. V. Frequency and distribution of rare electrophoretic mobility variants in a population of human newborns in Ann Arbor, Michigan. Ann Hum Genet. 1987 Oct;51(Pt 4):303–316. doi: 10.1111/j.1469-1809.1987.tb01065.x. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Gershowitz H., Mohrenweiser H. W., Amos B., Kostyu D. D., Salzano F. M., Mestriner M. A., Lawrence D., Simões A. L., Smouse P. E. Genetic studies on the Ticuna, an enigmatic tribe of Central Amazonas. Ann Hum Genet. 1980 Jul;44(Pt 1):37–54. doi: 10.1111/j.1469-1809.1980.tb00944.x. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Gershowitz H., Spielman R. S., Migliazza E. C., Salzano F. M., Oliver W. J. Genetic studies of the Macushi and Wapishana Indians. II. Data on 12 genetic polymorphisms of the red cell and serum proteins: gene flor between the tribes. Hum Genet. 1977 Jun 30;37(2):207–219. doi: 10.1007/BF00393584. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Mohrenweiser H. W., Gershowitz H. A pilot study of the use of placental cord blood samples in monitoring for mutational events. Mutat Res. 1988 Mar;204(3):365–377. doi: 10.1016/0165-1218(88)90035-3. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Mohrenweiser H. W., Rothman E. D., Naidu J. M. A revised indirect estimate of mutation rates in Amerindians. Am J Hum Genet. 1986 May;38(5):649–666. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Tanis R. J., Migliazza E. C., Spielman R. S., Salzano F., Oliver W. J., Morrow M., Bachofer S. Genetic studies of the Macushi and Wapishana Indians. I. Rare genetic variants and a "private polymorphism' of esterase A. Hum Genet. 1977 Apr 7;36(1):81–107. doi: 10.1007/BF00390440. [DOI] [PubMed] [Google Scholar]
- Neel J. V. The population structure of an Amerindian tribe, the Yanomama. Annu Rev Genet. 1978;12:365–413. doi: 10.1146/annurev.ge.12.120178.002053. [DOI] [PubMed] [Google Scholar]
- Nei M., Chakraborty R., Fuerst P. A. Infinite allele model with varying mutation rate. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4164–4168. doi: 10.1073/pnas.73.11.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Feldman M. W. Identity of genes by descent within and between populations under mutation and migration pressures. Theor Popul Biol. 1972 Dec;3(4):460–465. doi: 10.1016/0040-5809(72)90017-2. [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Smouse P. E., Neel J. V. The genetic structure of a tribal population, the Yanomama Indians. XV. Patterns inferred by autocorrelation analysis. Genetics. 1986 Sep;114(1):259–287. doi: 10.1093/genetics/114.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman R. S., Migliazza E. C., Neel J. V. Regional linguistic and genetic differences among Yanomama indians. Science. 1974 May 10;184(4137):637–644. doi: 10.1126/science.184.4137.637. [DOI] [PubMed] [Google Scholar]
- Tanis R. J., Neel J. V., Dovey H., Morrow M. The genetic structure of a tribal population, the Yanomama Indians. IX. Gene frequencies for 18 serum protein and erythrocyte enzyme systems in the Yanomama and five neighboring tribes: nine new variants. Am J Hum Genet. 1973 Nov;25(6):655–676. [PMC free article] [PubMed] [Google Scholar]
- Tanis R. J., Neel J. V., Torres de Arauz R. Two more "private" polymorphisms of Amerindian tribes: LDHb GUA-1 and ACP1 B GUA-1 in the Guaymi in Panama. Am J Hum Genet. 1977 Sep;29(5):419–430. [PMC free article] [PubMed] [Google Scholar]
- Wurzinger K. H., Mohrenweiser H. W. Studies on the genetic and non-genetic (physiological) variation of human erythrocyte glutamic oxaloacetic transaminase. Ann Hum Genet. 1982 Jul;46(Pt 3):191–201. doi: 10.1111/j.1469-1809.1982.tb00711.x. [DOI] [PubMed] [Google Scholar]


