Abstract
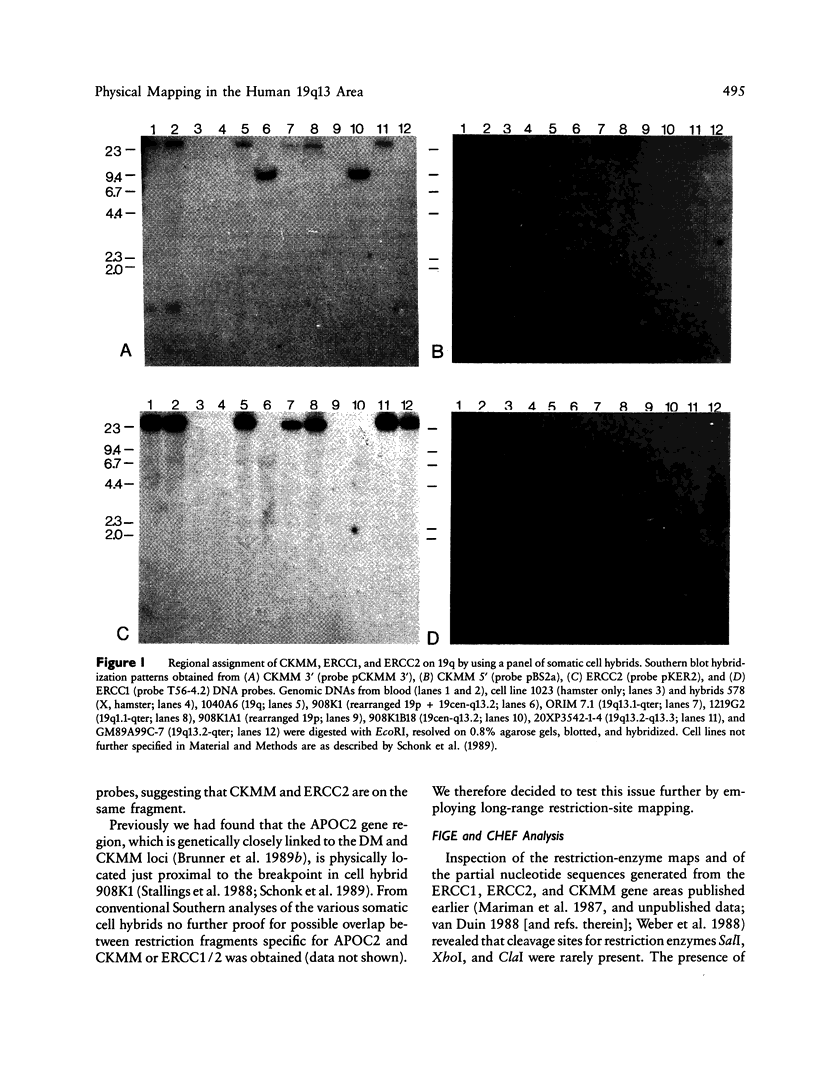
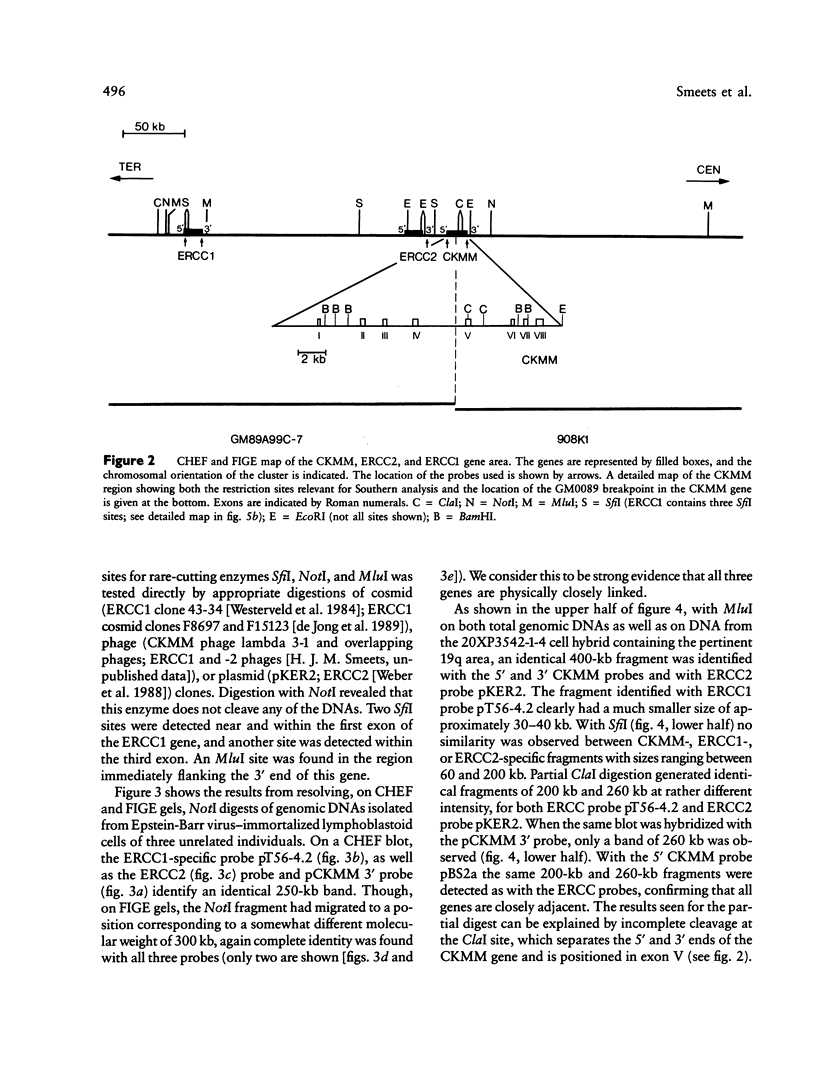
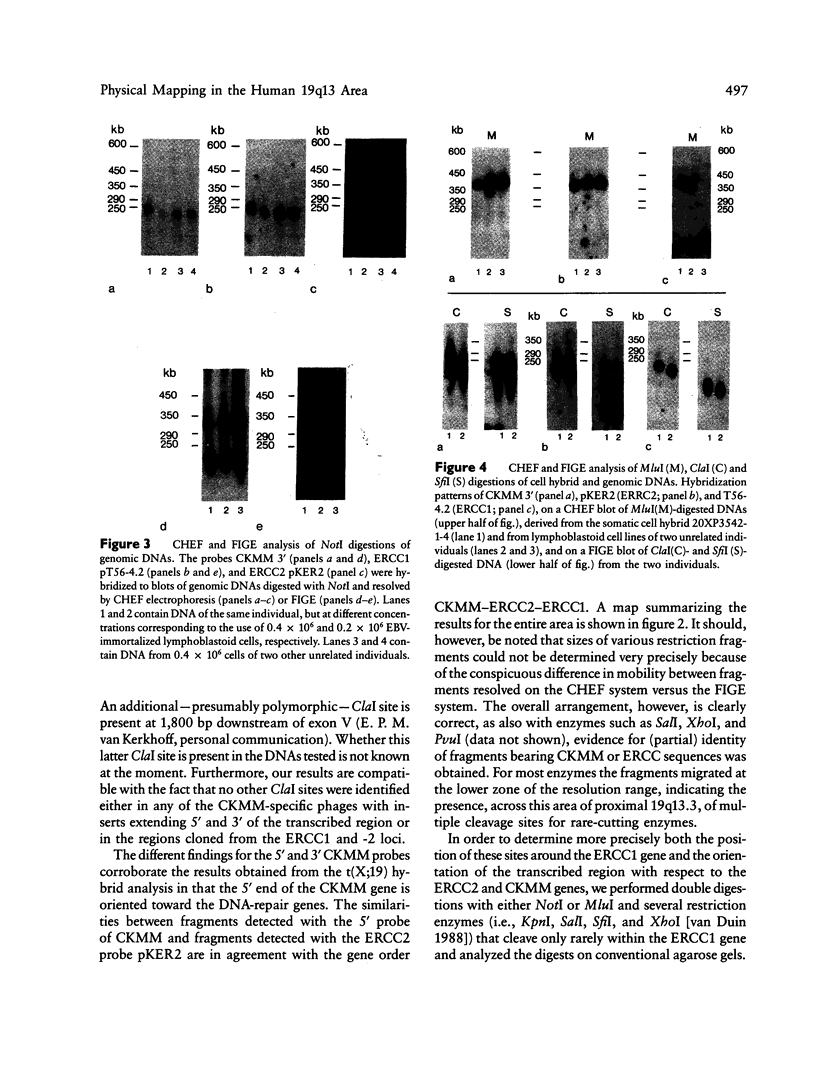
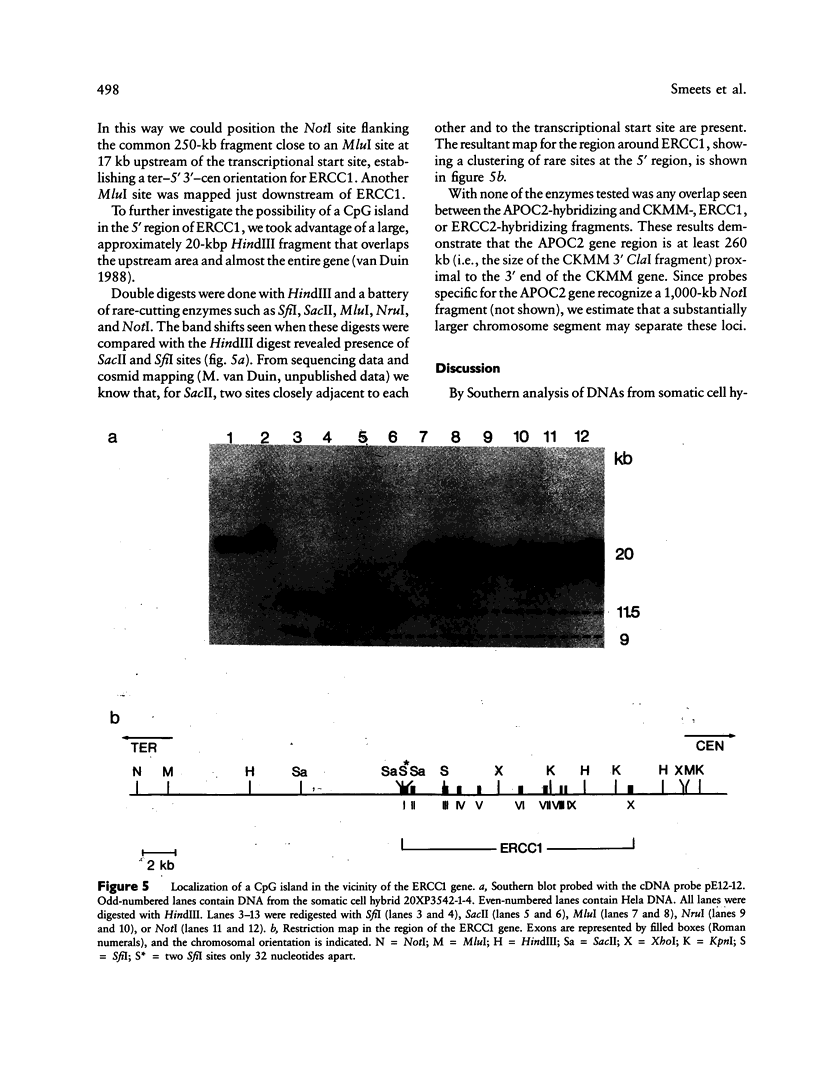
We report on the physical ordering of genes in a relatively small area of chromosome 19, segment q13, containing the locus for myotonic dystrophy (DM), the most frequent heritable muscular dystrophy of adulthood in man. DNAs from somatic cell hybrids with der 19q products that carry a breakpoint across the muscle-specific creatine kinase (CKMM) gene were analyzed by Southern blotting using probes for CKMM, APOC2, and the repair genes ERCC1 and ERCC2. Results were combined with data from CHEF and field inversion-gel-electrophoresis separation of large-sized DNA restriction fragments to establish a map localizing both DNA-repair genes and the CKMM gene within the same 250 kb of DNA, the order being cen–CKMM–ERCC2–ERCC1–ter, with APOC2 being at more than 260 kb proximal to CKMM. Transcriptional start sites of the CKMM and DNA-repair genes are all on the telomeric side of the genes. Our results provide a framework for the construction of a larger physical map of the area, which will facilitate the search for the DM gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Gottesman M. Promoter occlusion: transcription through a promoter may inhibit its activity. Cell. 1982 Jul;29(3):939–944. doi: 10.1016/0092-8674(82)90456-1. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Korneluk R. G., Coerwinkel-Driessen M., MacKenzie A., Smeets H., Lambermon H. M., van Oost B. A., Wieringa B., Ropers H. H. Myotonic dystrophy is closely linked to the gene for muscle-type creatine kinase (CKMM). Hum Genet. 1989 Mar;81(4):308–310. doi: 10.1007/BF00283680. [DOI] [PubMed] [Google Scholar]
- Brunner H. G., Smeets H., Lambermon H. M., Coerwinkel-Driessen M., van Oost B. A., Wieringa B., Ropers H. H. A multipoint linkage map around the locus for myotonic dystrophy on chromosome 19. Genomics. 1989 Oct;5(3):589–595. doi: 10.1016/0888-7543(89)90027-x. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Coerwinkel-Driessen M., Schepens J., van Zandvoort P., van Oost B., Mariman E., Wieringa B. NcoI RFLP at the creatine kinase-muscle type gene locus (CKMM, chromosome 19). Nucleic Acids Res. 1988 Sep 12;16(17):8743–8743. doi: 10.1093/nar/16.17.8743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers F. P., van de Pol D. J., Diergaarde P. J., Wieringa B., Nussbaum R. L., Schwartz M., Ropers H. H. Physical fine mapping of the choroideremia locus using Xq21 deletions associated with complex syndromes. Genomics. 1989 Jan;4(1):41–46. doi: 10.1016/0888-7543(89)90312-1. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gardiner K., Laas W., Patterson D. Fractionation of large mammalian DNA restriction fragments using vertical pulsed-field gradient gel electrophoresis. Somat Cell Mol Genet. 1986 Mar;12(2):185–195. doi: 10.1007/BF01560665. [DOI] [PubMed] [Google Scholar]
- Hulsebos T., Schonk D., van Dalen I., Coerwinkel-Driessen M., Schepens J., Ropers H. H., Wieringa B. Isolation and characterization of alphoid DNA sequences specific for the pericentric regions of chromosomes 4, 5, 9, and 19. Cytogenet Cell Genet. 1988;47(3):144–148. doi: 10.1159/000132533. [DOI] [PubMed] [Google Scholar]
- Hulsebos T., Wieringa B., Hochstenbach R., Smeets D., Schepens J., Oerlemans F., Zimmer J., Ropers H. H. Toward early diagnosis of myotonic dystrophy: construction and characterization of a somatic cell hybrid with a single human der(19) chromosome. Cytogenet Cell Genet. 1986;43(1-2):47–56. doi: 10.1159/000132297. [DOI] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. B., Villnave C. A., Singer R. H. Sensitive, high-resolution chromatin and chromosome mapping in situ: presence and orientation of two closely integrated copies of EBV in a lymphoma line. Cell. 1988 Jan 15;52(1):51–61. doi: 10.1016/0092-8674(88)90530-2. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Ryan D., Jr, Pericak-Vance M. A. Report of the committee on the genetic constitution of chromosomes 18 and 19. Cytogenet Cell Genet. 1989;51(1-4):338–357. doi: 10.1159/000132798. [DOI] [PubMed] [Google Scholar]
- Mariman E. C., Broers C. A., Claesen C. A., Tesser G. I., Wieringa B. Structure and expression of the human creatine kinase B gene. Genomics. 1987 Oct;1(2):126–137. doi: 10.1016/0888-7543(87)90004-8. [DOI] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Hellkuhl B., Grzeschik K. H., Shapiro L. J. Expression of an X-linked gene from an inactive human X chromosome in mouse-human hybrid cells: further evidence for the noninactivation of the steroid sulfatase locus in man. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6759–6763. doi: 10.1073/pnas.77.11.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., O'Connell P., Leppert M., Lalouel J. M., White R. A primary map of ten DNA markers and two serological markers for human chromosome 19. Genomics. 1988 Jul;3(1):67–71. doi: 10.1016/0888-7543(88)90161-9. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Landegent J., Collins C., Fuscoe J., Segraves R., Lucas J., Gray J. Fluorescence in situ hybridization with human chromosome-specific libraries: detection of trisomy 21 and translocations of chromosome 4. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9138–9142. doi: 10.1073/pnas.85.23.9138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schonk D., Coerwinkel-Driessen M., van Dalen I., Oerlemans F., Smeets B., Schepens J., Hulsebos T., Cockburn D., Boyd Y., Davis M. Definition of subchromosomal intervals around the myotonic dystrophy gene region at 19q. Genomics. 1989 Apr;4(3):384–396. doi: 10.1016/0888-7543(89)90346-7. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Smeets H. J., Brunner H. G., Ropers H. H., Wieringa B. Use of variable simple sequence motifs as genetic markers: application to study of myotonic dystrophy. Hum Genet. 1989 Oct;83(3):245–251. doi: 10.1007/BF00285165. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Olson E., Strauss A. W., Thompson L. H., Bachinski L. L., Siciliano M. J. Human creatine kinase genes on chromosomes 15 and 19, and proximity of the gene for the muscle form to the genes for apolipoprotein C2 and excision repair. Am J Hum Genet. 1988 Aug;43(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerveld A., Hoeijmakers J. H., van Duin M., de Wit J., Odijk H., Pastink A., Wood R. D., Bootsma D. Molecular cloning of a human DNA repair gene. Nature. 1984 Aug 2;310(5976):425–429. doi: 10.1038/310425a0. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Brunner H., Hulsebos T., Schonk D., Ropers H. H. Genetic and physical demarcation of the locus for dystrophia myotonica. Adv Neurol. 1988;48:47–69. [PubMed] [Google Scholar]