Abstract
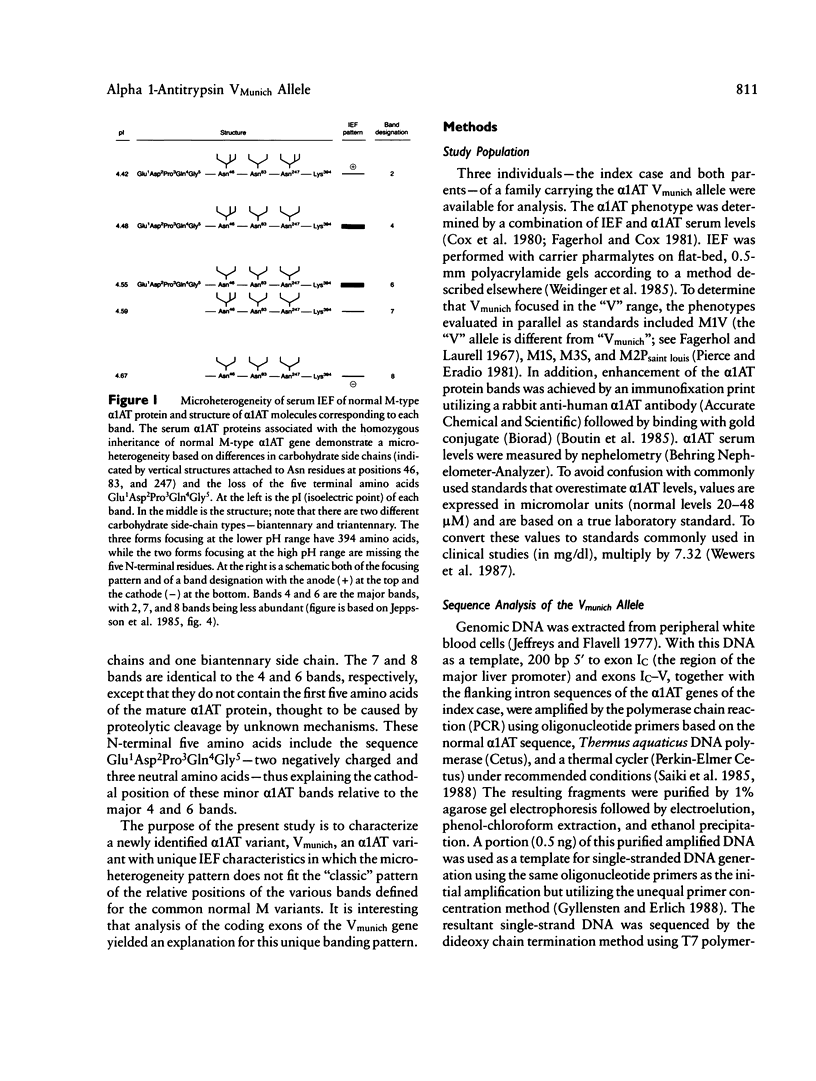
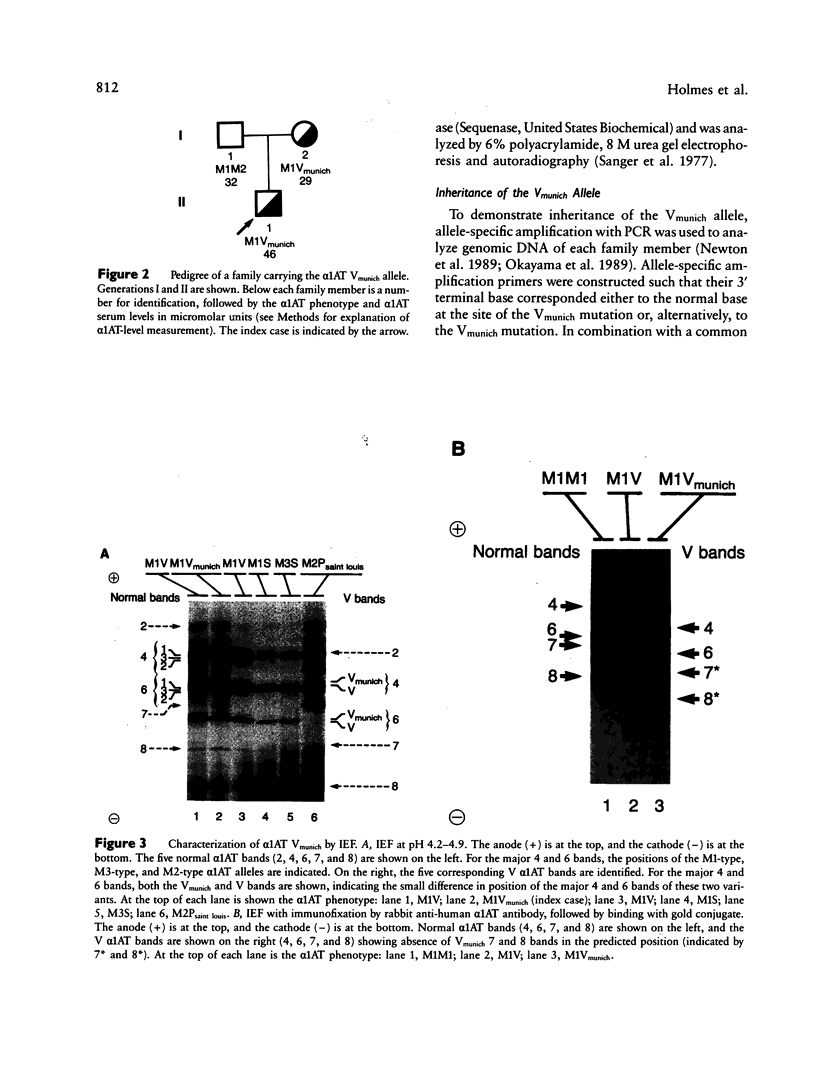
alpha 1-Antitrypsin (alpha 1AT), the major serum inhibitor of neutrophil elastase, is a highly polymorphic protein associated with isoelectric focusing (IEF) patterns typical for each variant. alpha 1AT Vmunich, a previously unreported normal alpha 1AT variant, has a unique IEF banding pattern in which the 7 and 8 alpha 1AT protein bands focus with the normal M-type 7 and 8 bands, despite the fact that the major fraction of the Vmunich protein focuses in the "V" region of the IEF gel. To characterize the molecular basis of this variant and its unique IEF pattern, DNA sequence analysis of the coding exons of the Vmunich alpha 1AT gene was carried out using the polymerase chain reaction. The Vmunich allele differed from the common normal M1(Val213) alpha 1AT allele by a single nucleotide substitution of cytosine for adenosine, with the resultant amino acid change Asp2 GAT----Ala GCT. Inheritance of the allele was confirmed by family analysis using allele-specific amplification with the polymerase chain reaction. The Asp2----Ala mutation explains the cathodal position of the Vmunich protein on IEF, as there is a substitution of a negatively charged amino acid by a neutral one.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF
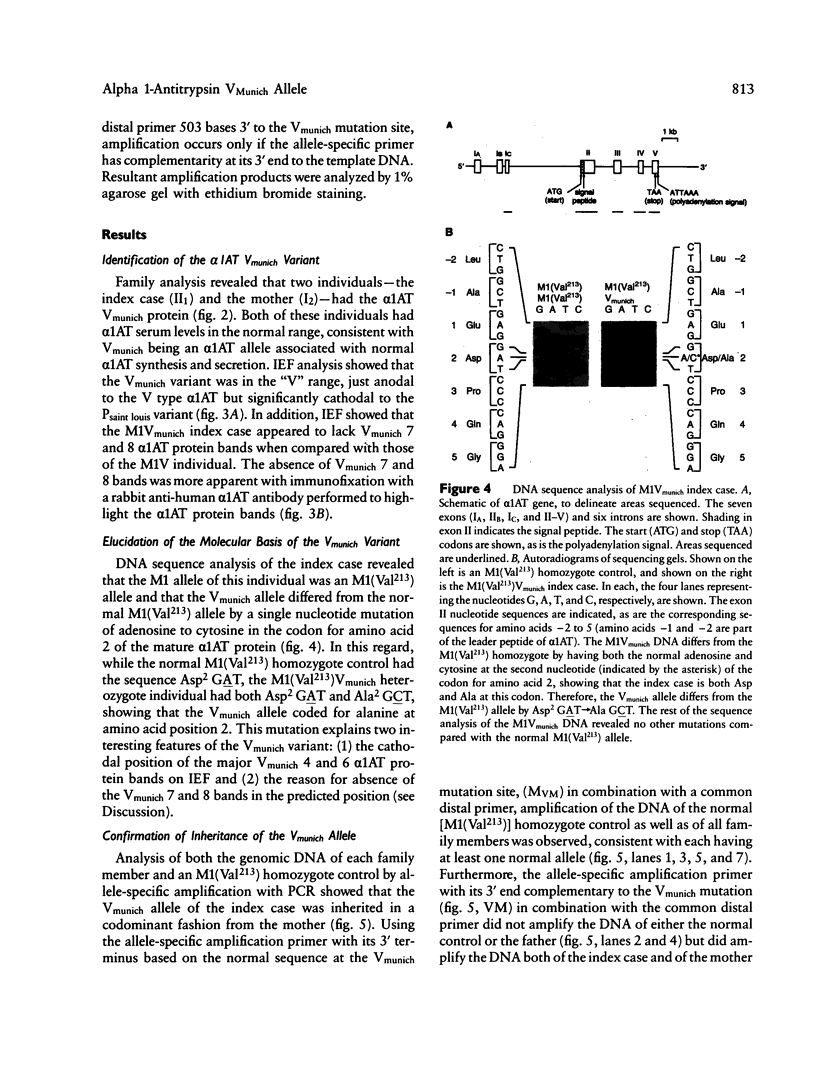
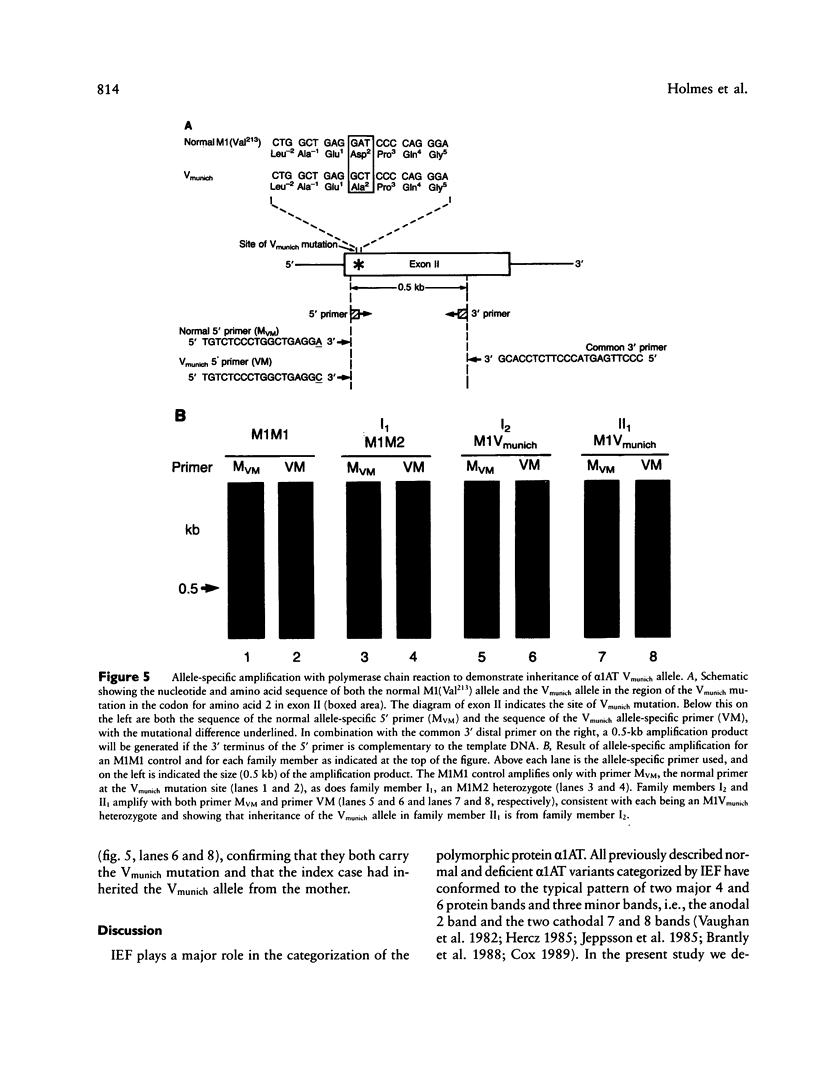


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boutin B., Feng S. H., Arnaud P. The genetic polymorphism of alpha 2-HS glycoprotein: study by ultrathin-layer isoelectric focusing and immunoblot. Am J Hum Genet. 1985 Nov;37(6):1098–1105. [PMC free article] [PubMed] [Google Scholar]
- Brantly M., Nukiwa T., Crystal R. G. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988 Jun 24;84(6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Brantly M. L., Hubbard R. C., Curiel D. T., States D. J., Holmes M. D. The alpha 1-antitrypsin gene and its mutations. Clinical consequences and strategies for therapy. Chest. 1989 Jan;95(1):196–208. doi: 10.1378/chest.95.1.196. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Fagerhol M. K., Laurell C. B. The polymorphism of "prealbumins" and alpha-1-antitrypsin in human sera. Clin Chim Acta. 1967 May;16(2):199–203. doi: 10.1016/0009-8981(67)90181-7. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercz A. Proteolytic cleavages in alpha 1-antitrypsin and microheterogeneity. Biochem Biophys Res Commun. 1985 Apr 16;128(1):199–203. doi: 10.1016/0006-291x(85)91664-x. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Jeppsson J. O., Lilja H., Johansson M. Isolation and characterization of two minor fractions of alpha 1-antitrypsin by high-performance liquid chromatographic chromatofocusing. J Chromatogr. 1985 Jun 26;327:173–177. doi: 10.1016/s0021-9673(01)81646-0. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Newton C. R., Graham A., Heptinstall L. E., Powell S. J., Summers C., Kalsheker N., Smith J. C., Markham A. F. Analysis of any point mutation in DNA. The amplification refractory mutation system (ARMS). Nucleic Acids Res. 1989 Apr 11;17(7):2503–2516. doi: 10.1093/nar/17.7.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Curiel D. T., Brantly M. L., Holmes M. D., Crystal R. G. Rapid, nonradioactive detection of mutations in the human genome by allele-specific amplification. J Lab Clin Med. 1989 Aug;114(2):105–113. [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. A., Eradio B. MPsaintlouis: a new antitrypsin phenotype. Hum Hered. 1981;31(1):35–38. doi: 10.1159/000153173. [DOI] [PubMed] [Google Scholar]
- Rabin M., Watson M., Kidd V., Woo S. L., Breg W. R., Ruddle F. H. Regional location of alpha 1-antichymotrypsin and alpha 1-antitrypsin genes on human chromosome 14. Somat Cell Mol Genet. 1986 Mar;12(2):209–214. doi: 10.1007/BF01560668. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Lorier M. A., Carrell R. W. alpha 1-Antitrypsin microheterogeneity. Isolation and physiological significance of isoforms. Biochim Biophys Acta. 1982 Mar 4;701(3):339–345. doi: 10.1016/0167-4838(82)90237-0. [DOI] [PubMed] [Google Scholar]
- Weidinger S., Jahn W., Cujnik F., Schwarzfischer F. Alpha-1-antitrypsin: evidence for a fifth PI M subtype and a new deficiency allele PI*Z augsburg. Hum Genet. 1985;71(1):27–29. doi: 10.1007/BF00295662. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]