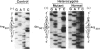Abstract
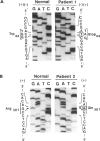
Efforts were directed to identify the specific mutations in the alpha-galactosidase A (alpha-Gal A) gene which cause Fabry disease in families of Japanese origin. By polymerase-chain-reaction-amplification of DNA from reverse-transcribed mRNA and genomic DNA, different point mutations were found in two unrelated Fabry hemizygotes. A hemizygote with classic disease manifestations and no detectable alpha-Gal A activity had a G-to-A transition in exon 1 (codon 44) which substituted a termination codon (TAG) for a tryptophan codon (TGG) and created an NheI restriction site. This point mutation would predict a truncated alpha-Gal A polypeptide, consistent with the observed absence of enzymatic activity and a classic Fabry phenotype. In an unrelated Japanese hemizygote who had an atypical clinical course characterized by late-onset cardiac involvement and significant residual alpha-Gal activity, a G-to-A transition in exon 6 (codon 301) resulted in the replacement of a glutamine for an arginine residue. This amino acid substitution apparently altered the properties of the enzyme such that sufficient enzymatic activity was retained to markedly alter the disease course. Identification of these mutations permitted accurate molecular heterozygote diagnosis in these families.
Full text
PDF


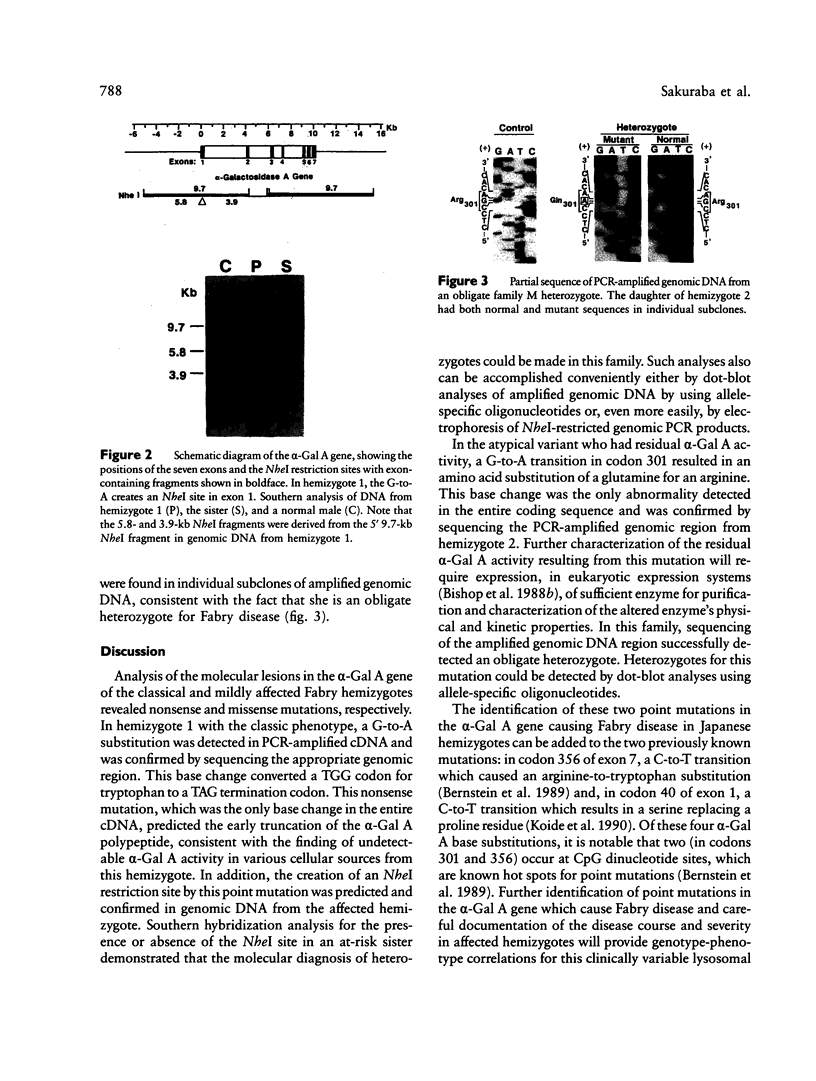

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Bach G., Rosenmann E., Karni A., Cohen T. Pseudodeficiency of alpha-galactosidase A. Clin Genet. 1982 Jan;21(1):59–64. [PubMed] [Google Scholar]
- Bernstein H. S., Bishop D. F., Astrin K. H., Kornreich R., Eng C. M., Sakuraba H., Desnick R. J. Fabry disease: six gene rearrangements and an exonic point mutation in the alpha-galactosidase gene. J Clin Invest. 1989 Apr;83(4):1390–1399. doi: 10.1172/JCI114027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Calhoun D. H., Bernstein H. S., Hantzopoulos P., Quinn M., Desnick R. J. Human alpha-galactosidase A: nucleotide sequence of a cDNA clone encoding the mature enzyme. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4859–4863. doi: 10.1073/pnas.83.13.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. F., Kornreich R., Desnick R. J. Structural organization of the human alpha-galactosidase A gene: further evidence for the absence of a 3' untranslated region. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3903–3907. doi: 10.1073/pnas.85.11.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. T., Knaack J., Crawhall J. C., Wolfe L. S. Ceramide trihexosidosis (fabry's disease) without skin lesions. N Engl J Med. 1971 Feb 4;284(5):233–235. doi: 10.1056/NEJM197102042840503. [DOI] [PubMed] [Google Scholar]
- Desnick R. J., Bernstein H. S., Astrin K. H., Bishop D. F. Fabry disease: molecular diagnosis of hemizygotes and heterozygotes. Enzyme. 1987;38(1-4):54–64. doi: 10.1159/000469190. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kira J., Shinnoh N., Goto I., Kuroiwa Y. Fabry's disease with partially deficient hydrolysis of ceramide trihexoside. J Neurol Sci. 1985 Feb;67(2):179–185. doi: 10.1016/0022-510x(85)90114-5. [DOI] [PubMed] [Google Scholar]
- Koide T., Ishiura M., Iwai K., Inoue M., Kaneda Y., Okada Y., Uchida T. A case of Fabry's disease in a patient with no alpha-galactosidase A activity caused by a single amino acid substitution of Pro-40 by Ser. FEBS Lett. 1990 Jan 1;259(2):353–356. doi: 10.1016/0014-5793(90)80046-l. [DOI] [PubMed] [Google Scholar]
- Kornreich R., Bishop D. F., Desnick R. J. Alpha-galactosidase A gene rearrangements causing Fabry disease. Identification of short direct repeats at breakpoints in an Alu-rich gene. J Biol Chem. 1990 Jun 5;265(16):9319–9326. [PubMed] [Google Scholar]
- Kornreich R., Desnick R. J., Bishop D. F. Nucleotide sequence of the human alpha-galactosidase A gene. Nucleic Acids Res. 1989 Apr 25;17(8):3301–3302. doi: 10.1093/nar/17.8.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYON M. F. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 1961 Apr 22;190:372–373. doi: 10.1038/190372a0. [DOI] [PubMed] [Google Scholar]
- Oshima A., Tsuji A., Nagao Y., Sakuraba H., Suzuki Y. Cloning, sequencing, and expression of cDNA for human beta-galactosidase. Biochem Biophys Res Commun. 1988 Nov 30;157(1):238–244. doi: 10.1016/s0006-291x(88)80038-x. [DOI] [PubMed] [Google Scholar]
- Romeo G., D'Urso M., Pisacane A., Blum E., De Falco A., Ruffilli A. Residual activity of alpha-galactosidase A in Fabry's disease. Biochem Genet. 1975 Oct;13(9-10):615–628. doi: 10.1007/BF00484919. [DOI] [PubMed] [Google Scholar]
- SWEELEY C. C., KLIONSKY B. FABRY'S DISEASE: CLASSIFICATION AS A SPHINGOLIPIDOSIS AND PARTIAL CHARACTERIZATION OF A NOVEL GLYCOLIPID. J Biol Chem. 1963 Sep;238:3148–3150. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]