Abstract
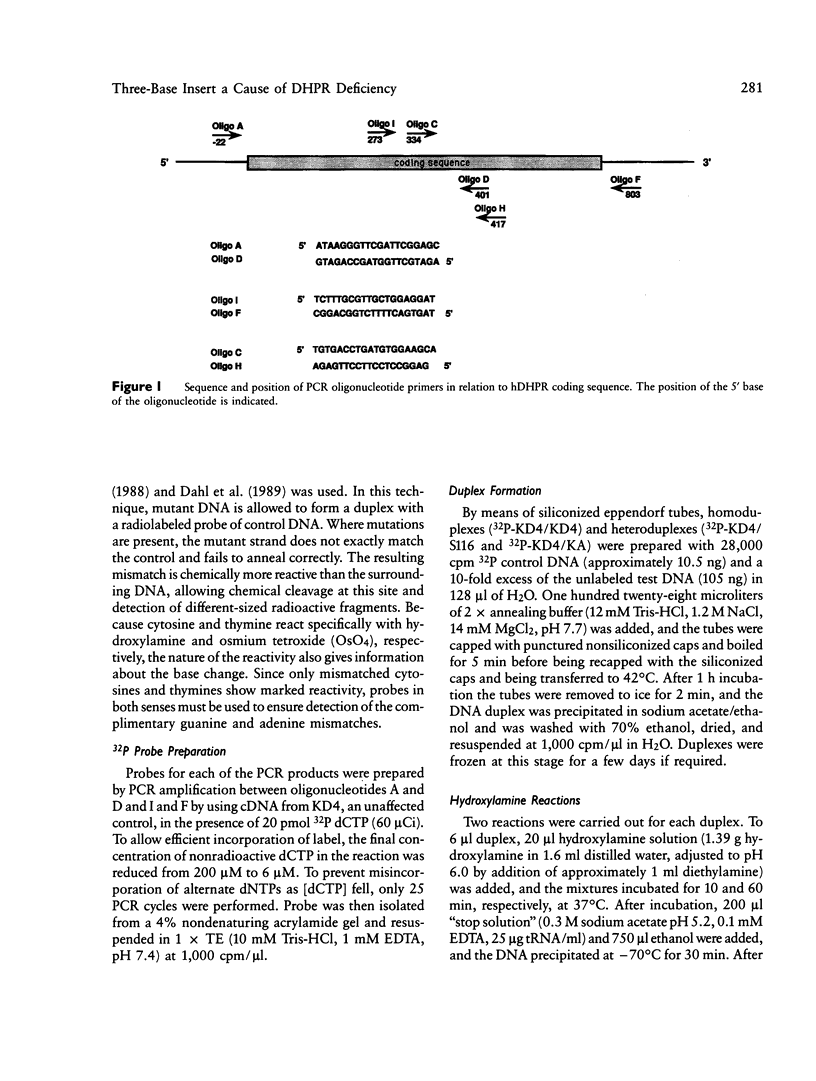
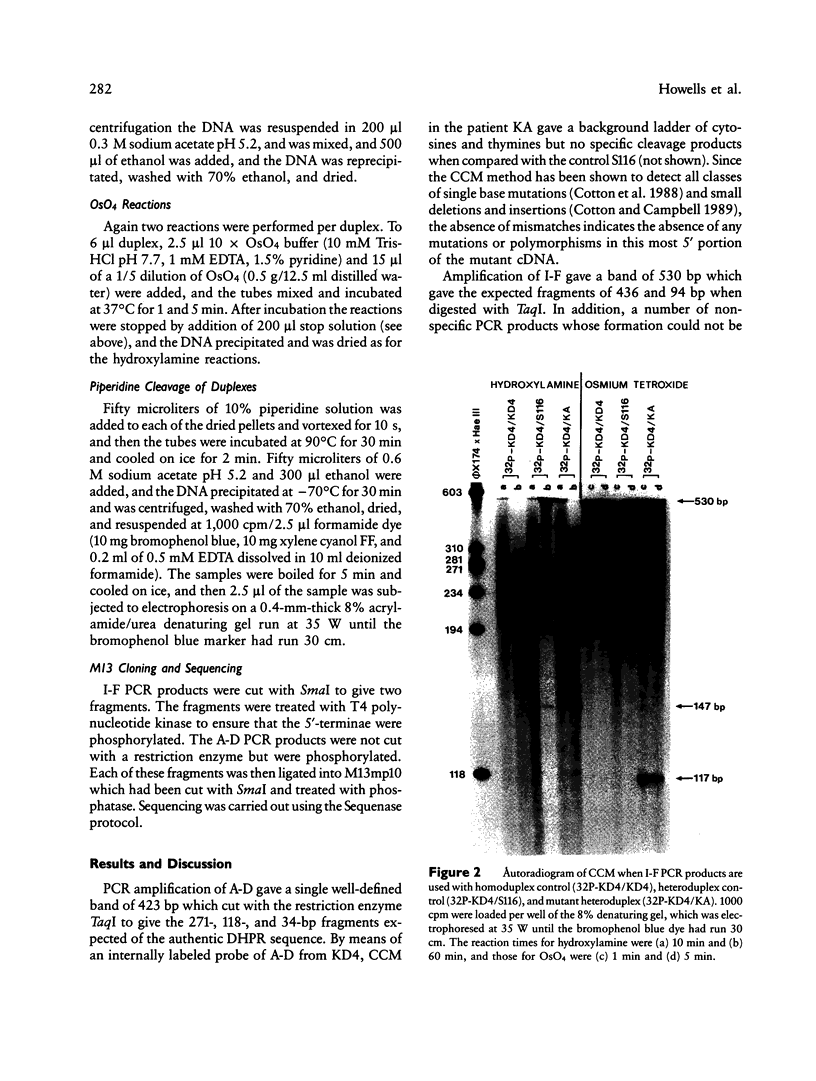
The mutation in a patient with dihydropteridine reductase deficiency has been located and characterized. Polymerase chain reaction (PCR) was used to amplify the coding sequence of human dihydropteridine reductase from the messenger RNA of skin fibroblasts. Chemical cleavage of mismatches indicated a mismatched thymine and cytosine at approximately 117 and 147 bases, respectively, from the end of the probe. Cloning and sequencing of the mutant PCR products revealed the insertion of the triplet ACT (threonine), after alanine 122 (base 390). Amplification of a small region around this mutation by using genomic DNA as the PCR target indicates that the mutation is completely within an exon. Unequal crossing-over at the second base in the preceding alanine codon and duplication of the bases CTA may be the mechanism of mutagenesis. The cleavage site 147 bases from the end of the probe corresponded to the conversion of guanine to adenine at base 420 (CTG to CTA) and does not alter the code for leucine. This change, which was also seen in another dihydropteridine reductase-deficient child and in a control subject probably represents a common neutral polymorphism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armarego W. L., Randles D., Waring P. Dihydropteridine reductase (DHPR), its cofactors, and its mode of action. Med Res Rev. 1984 Jul-Sep;4(3):267–321. doi: 10.1002/med.2610040302. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Campbell R. D. Chemical reactivity of matched cytosine and thymine bases near mismatched and unmatched bases in a heteroduplex between DNA strands with multiple differences. Nucleic Acids Res. 1989 Jun 12;17(11):4223–4233. doi: 10.1093/nar/17.11.4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine J. E., Hall E. S., Kaufman S. The isolation and characterization of dihydropteridine reductase from sheep liver. J Biol Chem. 1972 Oct 10;247(19):6082–6091. [PubMed] [Google Scholar]
- Dahl H. H., Hutchison W., McAdam W., Wake S., Morgan F. J., Cotton R. G. Human dihydropteridine reductase: characterisation of a cDNA clone and its use in analysis of patients with dihydropteridine reductase deficiency. Nucleic Acids Res. 1987 Mar 11;15(5):1921–1932. doi: 10.1093/nar/15.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Lamande S. R., Cotton R. G., Bateman J. F. Detection and localization of base changes in RNA using a chemical cleavage method. Anal Biochem. 1989 Dec;183(2):263–268. doi: 10.1016/0003-2697(89)90477-6. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Wake S., Cotton R. G., Danks D. M. The use of restriction fragment length polymorphisms in prenatal diagnosis of dihydropteridine reductase deficiency. J Med Genet. 1988 Jan;25(1):25–28. doi: 10.1136/jmg.25.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firgaira F. A., Choo K. H., Cotton R. G., Danks D. M. Heterogeneity of the molecular defect in human dihydropteridine reductase deficiency. Biochem J. 1981 Sep 15;198(3):677–682. doi: 10.1042/bj1980677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firgaira F. A., Cotton R. G., Danks D. M. Dihydropteridine reductase deficiency diagnosis by assays on peripheral blood cells. Lancet. 1980 Jan 19;1(8160):160–160. doi: 10.1016/s0140-6736(80)90648-0. [DOI] [PubMed] [Google Scholar]
- Firgaira F. A., Cotton R. G., Danks D. M., Fowler K., Lipson A., Yu J. S. Prenatal determination of dihydropteridine reductase in a normal fetus at risk for malignant hyperphenylalaninemia. Prenat Diagn. 1983 Jan;3(1):7–11. doi: 10.1002/pd.1970030103. [DOI] [PubMed] [Google Scholar]
- Hosoda S., Glick D. Studies in histochemistry. LXXIX. Properties of tryptophan hydroxylase from neoplastic murine mast cells. J Biol Chem. 1966 Jan 10;241(1):192–196. [PubMed] [Google Scholar]
- KAUFMAN S. A new cofactor required for the enzymatic conversion of phenylalanine to tyrosine. J Biol Chem. 1958 Feb;230(2):931–939. [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- Lai P. H., Pan Y. C., Gleisner J. M., Peterson D. L., Williams K. R., Blakley R. L. Structure of dihydrofolate reductase: primary sequence of the bovine liver enzyme. Biochemistry. 1982 Jul 6;21(14):3284–3294. doi: 10.1021/bi00257a006. [DOI] [PubMed] [Google Scholar]
- Lockyer J., Cook R. G., Milstien S., Kaufman S., Woo S. L., Ledley F. D. Structure and expression of human dihydropteridine reductase. Proc Natl Acad Sci U S A. 1987 May;84(10):3329–3333. doi: 10.1073/pnas.84.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaz M., Hoch J. A., Trach K. A., Hural J. A., Webber S., Whiteley J. M. Structural studies and isolation of cDNA clones providing the complete sequence of rat liver dihydropteridine reductase. J Biol Chem. 1987 Dec 5;262(34):16412–16416. [PubMed] [Google Scholar]
- Smith I., Howells D. W., Hyland K. Pteridines and mono-amines: relevance to neurological damage. Postgrad Med J. 1986 Feb;62(724):113–123. doi: 10.1136/pgmj.62.724.113. [DOI] [PMC free article] [PubMed] [Google Scholar]