Abstract
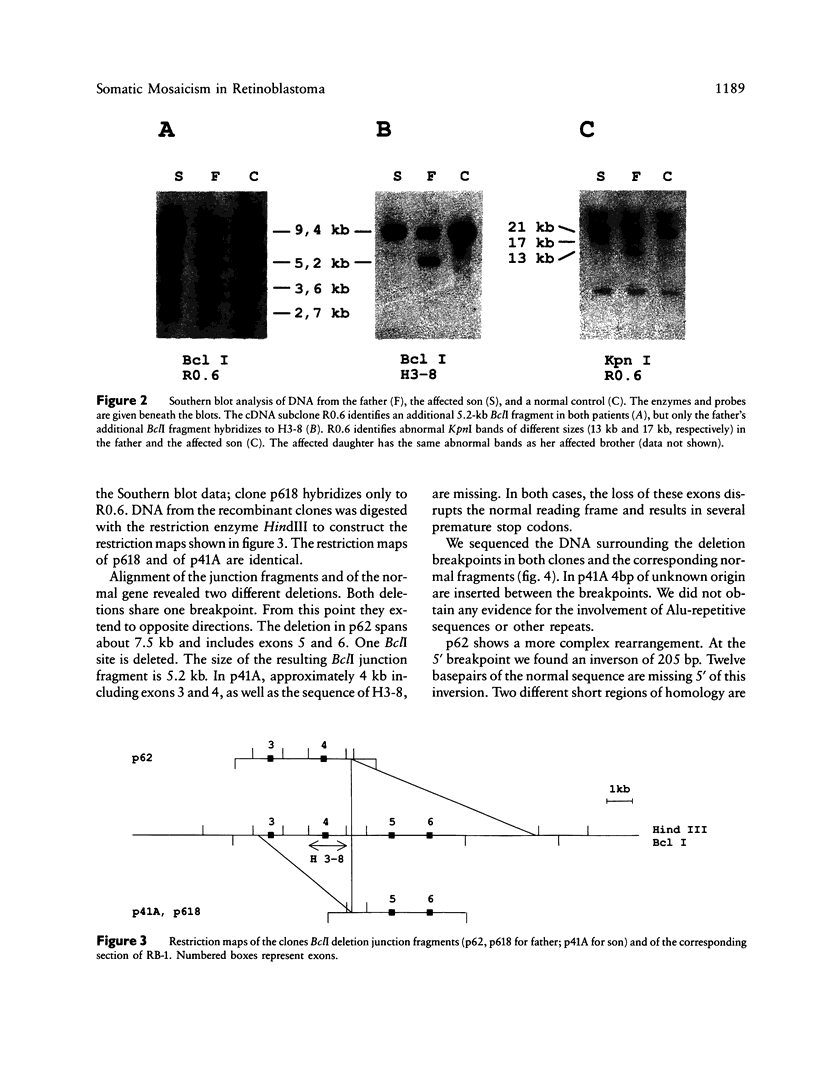
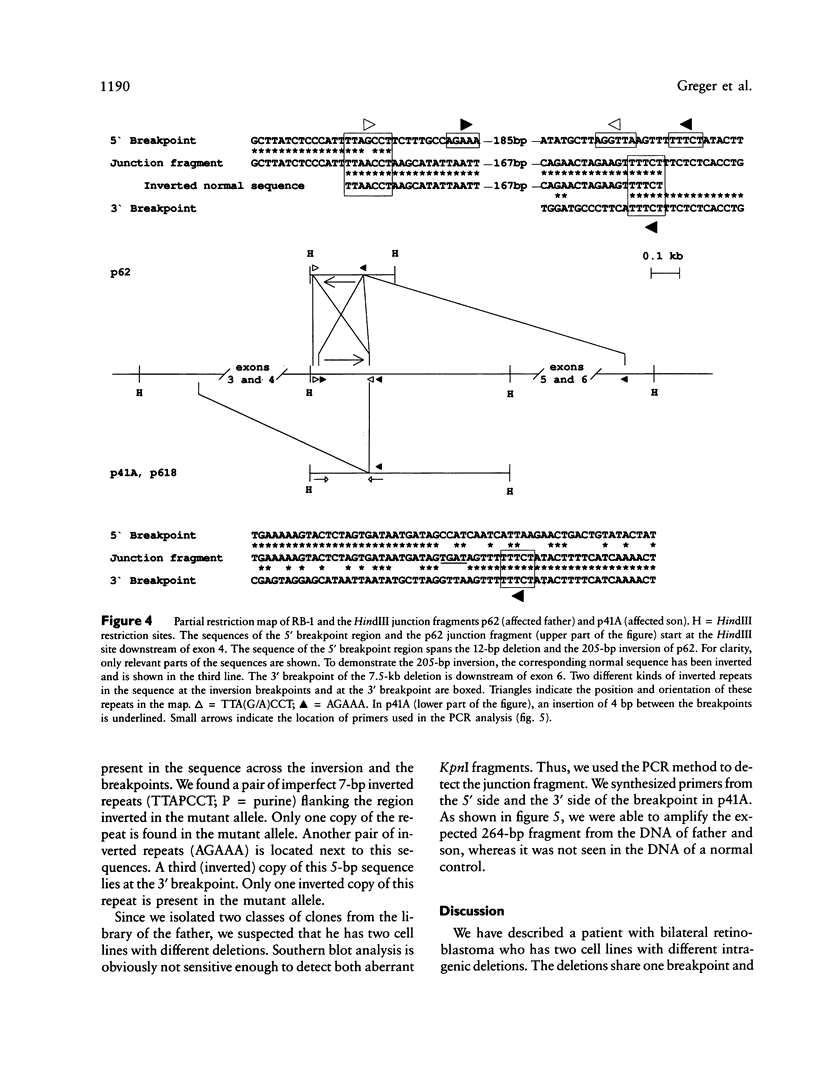
We describe two cell lines with different deletions of the retinoblastoma gene in a patient with bilateral retinoblastoma. This patient has transmitted the mutation less frequent in his lymphocytes to two affected children. We cloned, mapped, and sequenced the junction fragments of the two deletions and found that they share one breakpoint but extend into opposite directions. An insertion of 4 bp of unknown origin is present between the breakpoints in one of the deletions. The second deletion shows a more complex rearrangement, including an inversion at the 5' end. Short regions of homology were found at the breakpoints and flanking the inversion. These results support the notion that bilateral retinoblastoma may not only be due to a germ-line mutation but also to a postzygotic mutation leading to somatic mosaicism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Lee E. Y., To H., Young L. J., Sery T. W., Hayes R. C., Friedmann T., Lee W. H. Human retinoblastoma susceptibility gene: genomic organization and analysis of heterozygous intragenic deletion mutants. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2210–2214. doi: 10.1073/pnas.85.7.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunier D., Michel B., Ehrlich S. D. Copy choice illegitimate DNA recombination. Cell. 1988 Mar 25;52(6):883–892. doi: 10.1016/0092-8674(88)90430-8. [DOI] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J. M., Joyce J. M., Petersen R. A. Molecular detection of deletions involving band q14 of chromosome 13 in retinoblastomas. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7391–7394. doi: 10.1073/pnas.83.19.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Becker A. J., Gallie B. L. Identification of germline and somatic mutations affecting the retinoblastoma gene. Science. 1988 Sep 30;241(4874):1797–1800. doi: 10.1126/science.3175621. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Horowitz J. M., Gerber M. R., Wang X. F., Bogenmann E., Li F. P., Weinberg R. A. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9059–9063. doi: 10.1073/pnas.84.24.9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Murphree A. L., T'Ang A., Qian J., Hinrichs S. H., Benedict W. F. Structural evidence for the authenticity of the human retinoblastoma gene. Science. 1987 Jun 26;236(4809):1657–1661. doi: 10.1126/science.2885916. [DOI] [PubMed] [Google Scholar]
- Goddard A. D., Balakier H., Canton M., Dunn J., Squire J., Reyes E., Becker A., Phillips R. A., Gallie B. L. Infrequent genomic rearrangement and normal expression of the putative RB1 gene in retinoblastoma tumors. Mol Cell Biol. 1988 May;8(5):2082–2088. doi: 10.1128/mcb.8.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard A. D., Phillips R. A., Greger V., Passarge E., Höpping W., Zhu X. P., Gallie B. L., Horsthemke B. Use of the RB1 cDNA as a diagnostic probe in retinoblastoma families. Clin Genet. 1990 Feb;37(2):117–126. doi: 10.1111/j.1399-0004.1990.tb03488.x. [DOI] [PubMed] [Google Scholar]
- Hall J. G. Review and hypotheses: somatic mosaicism: observations related to clinical genetics. Am J Hum Genet. 1988 Oct;43(4):355–363. [PMC free article] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Beisiegel U., Dunning A., Havinga J. R., Williamson R., Humphries S. Unequal crossing-over between two alu-repetitive DNA sequences in the low-density-lipoprotein-receptor gene. A possible mechanism for the defect in a patient with familial hypercholesterolaemia. Eur J Biochem. 1987 Apr 1;164(1):77–81. doi: 10.1111/j.1432-1033.1987.tb10995.x. [DOI] [PubMed] [Google Scholar]
- Horsthemke B., Greger V., Barnert H. J., Höpping W., Passarge E. Detection of submicroscopic deletions and a DNA polymorphism at the retinoblastoma locus. Hum Genet. 1987 Jul;76(3):257–261. doi: 10.1007/BF00283619. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalande M., Dryja T. P., Schreck R. R., Shipley J., Flint A., Latt S. A. Isolation of human chromosome 13-specific DNA sequences cloned from flow sorted chromosomes and potentially linked to the retinoblastoma locus. Cancer Genet Cytogenet. 1984 Dec;13(4):283–295. doi: 10.1016/0165-4608(84)90073-6. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., To H., Shew J. Y., Bookstein R., Scully P., Lee W. H. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science. 1988 Jul 8;241(4862):218–221. doi: 10.1126/science.3388033. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Russell D. W., Goldstein J. L., Brown M. S. Exon-Alu recombination deletes 5 kilobases from the low density lipoprotein receptor gene, producing a null phenotype in familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3679–3683. doi: 10.1073/pnas.83.11.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Schneider W. J., Südhof T. C., Brown M. S., Goldstein J. L., Russell D. W. Mutation in LDL receptor: Alu-Alu recombination deletes exons encoding transmembrane and cytoplasmic domains. Science. 1985 Jan 11;227(4683):140–146. doi: 10.1126/science.3155573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckett W. P. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978 May;152(1):59–97. doi: 10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- McGee T. L., Yandell D. W., Dryja T. P. Structure and partial genomic sequence of the human retinoblastoma susceptibility gene. Gene. 1989 Aug 1;80(1):119–128. doi: 10.1016/0378-1119(89)90256-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro M. C., Andrade J. A., Erwenne C. M., Brunoni D. Bilateral retinoblastoma associated with 13q-mosaicism. Possible manifestation of a germinal mutation. Cancer Genet Cytogenet. 1988 Jun;32(2):169–175. doi: 10.1016/0165-4608(88)90279-8. [DOI] [PubMed] [Google Scholar]
- Soriano P., Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986 Jul 4;46(1):19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- Vogel F. Genetics of retinoblastoma. Hum Genet. 1979 Nov 1;52(1):1–54. doi: 10.1007/BF00284597. [DOI] [PubMed] [Google Scholar]
- Wiggs J., Nordenskjöld M., Yandell D., Rapaport J., Grondin V., Janson M., Werelius B., Petersen R., Craft A., Riedel K. Prediction of the risk of hereditary retinoblastoma, using DNA polymorphisms within the retinoblastoma gene. N Engl J Med. 1988 Jan 21;318(3):151–157. doi: 10.1056/NEJM198801213180305. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]