Abstract
Although of great clinical and biological importance, the role of genotype-diet interaction in lipoprotein metabolism and atherosclerosis is still poorly understood. We analyzed serum apolipoprotein A-I (apo A-I) concentrations of approximately 600 pedigreed baboons that were fed two dietary regimens: (1) a basal diet and (2) an atherogenic (high-cholesterol, saturated-fat) diet. Complex segregation analysis was performed separately for apo A-I concentrations in each dietary environment. A major locus model with a recessive allele for high levels of apo A-I and a polygenic component best fit the family data for both diets. Using bivariate segregation analysis, we showed that the major genes detected in the univariate analyses represent two distinct loci that act additively to determine apo A-I concentrations. These two loci accounted for approximately 40% of the total phenotypic variance in apo A-I levels in each dietary environment and were also responsible for 33% of the variation in apo A-I response to the atherogenic diet. Both major loci were influenced by genotype-diet interaction in which the two-locus genotypes exhibited heterogeneous responses to the atherogenic diet. Most genotypes responded to the atherogenic diet with an increase in apo A-I, but two genotypes showed a decrease that can be traced to the effect of one of the major loci. The presence of two major loci and genotype-diet interaction may be responsible for the equivocal results obtained in human pedigree studies of apo A-I.
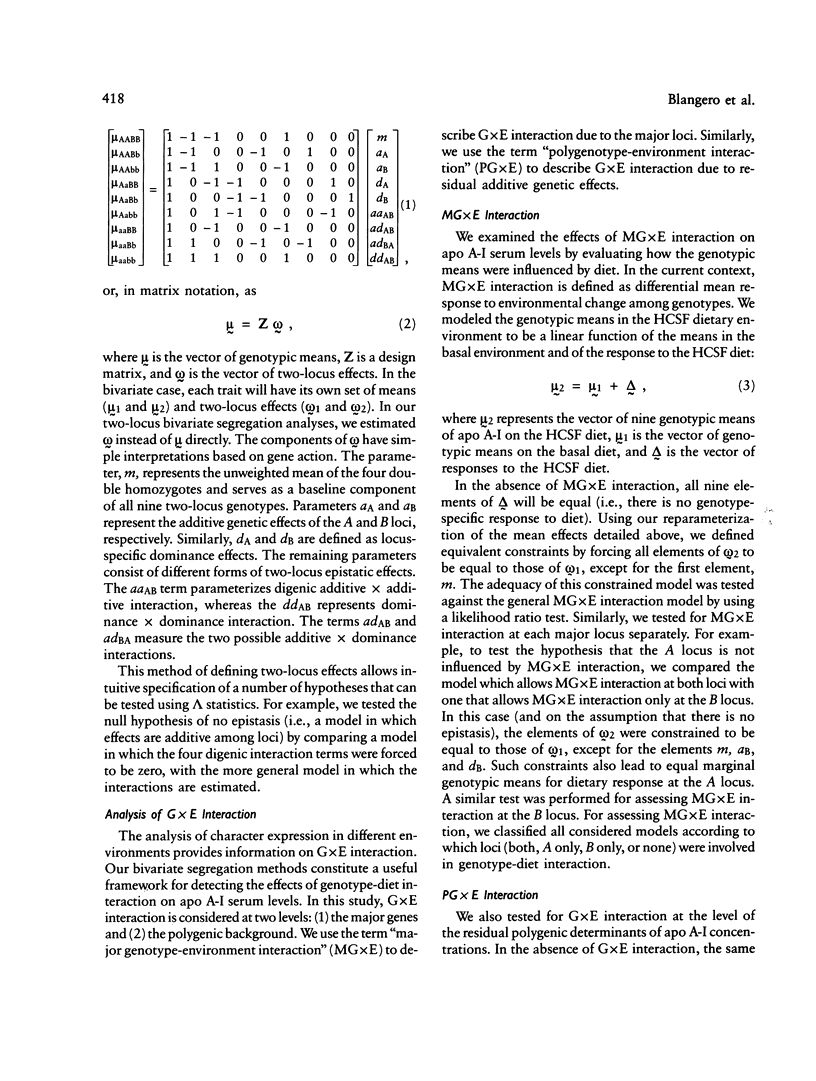
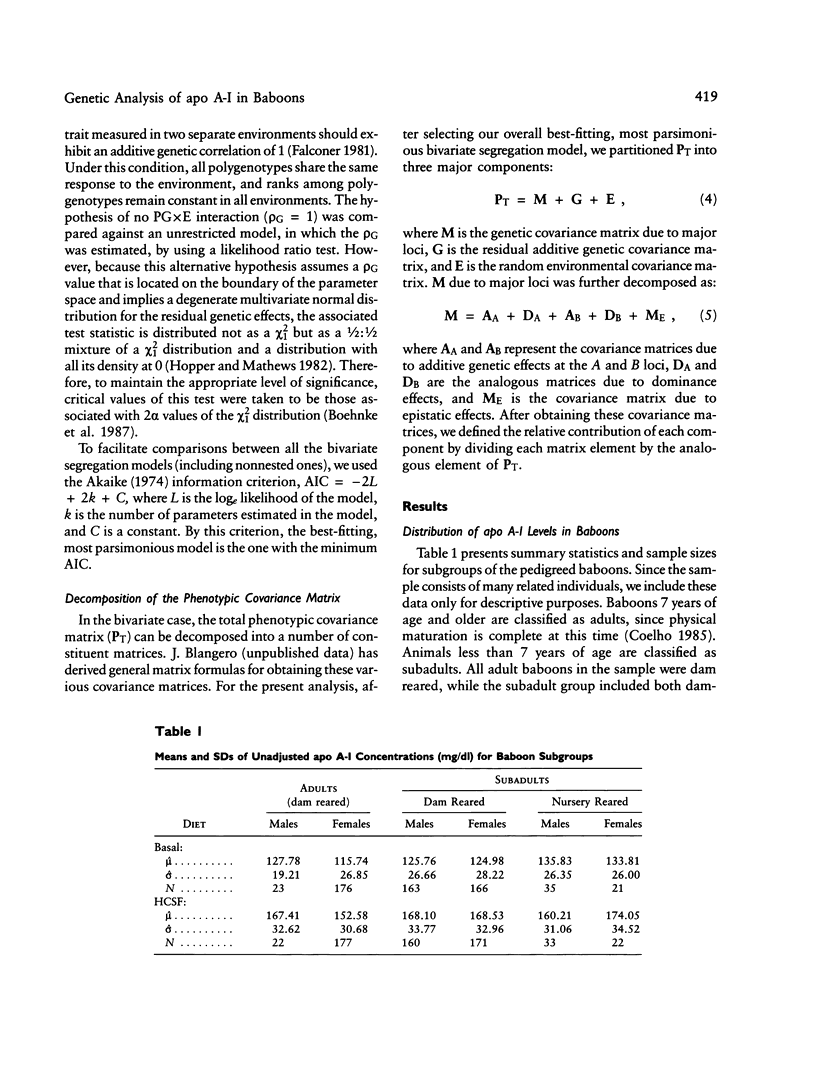
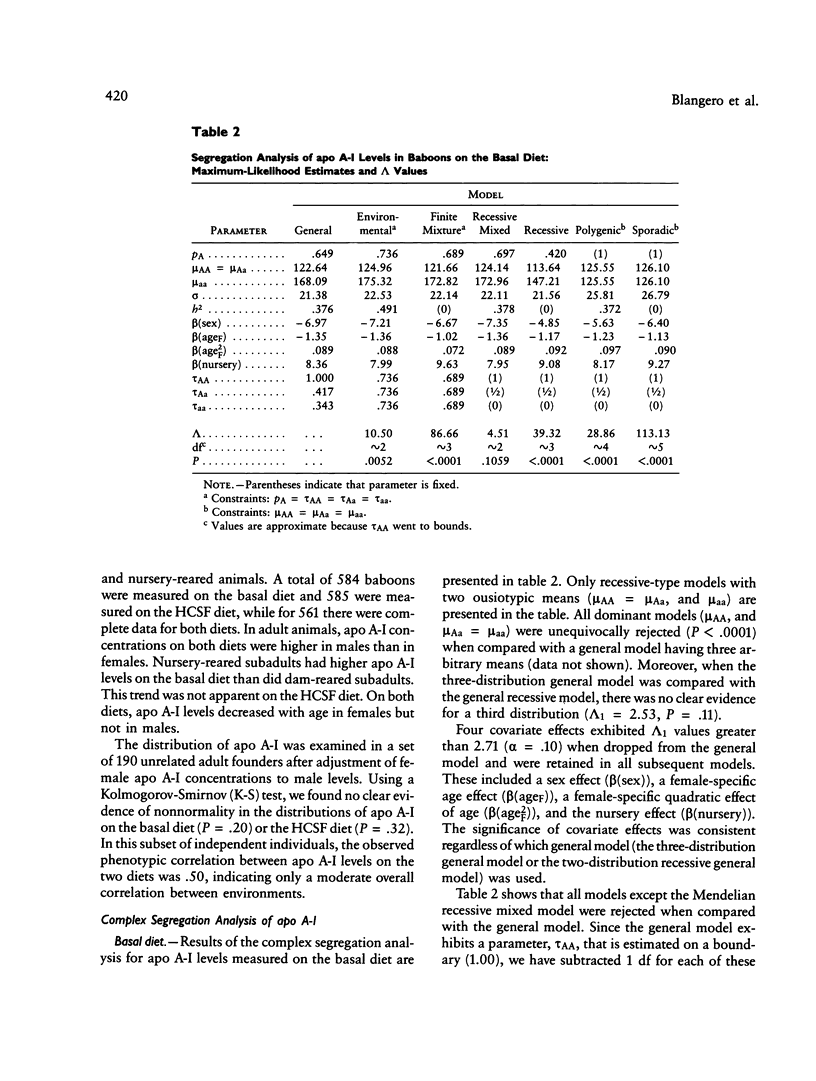
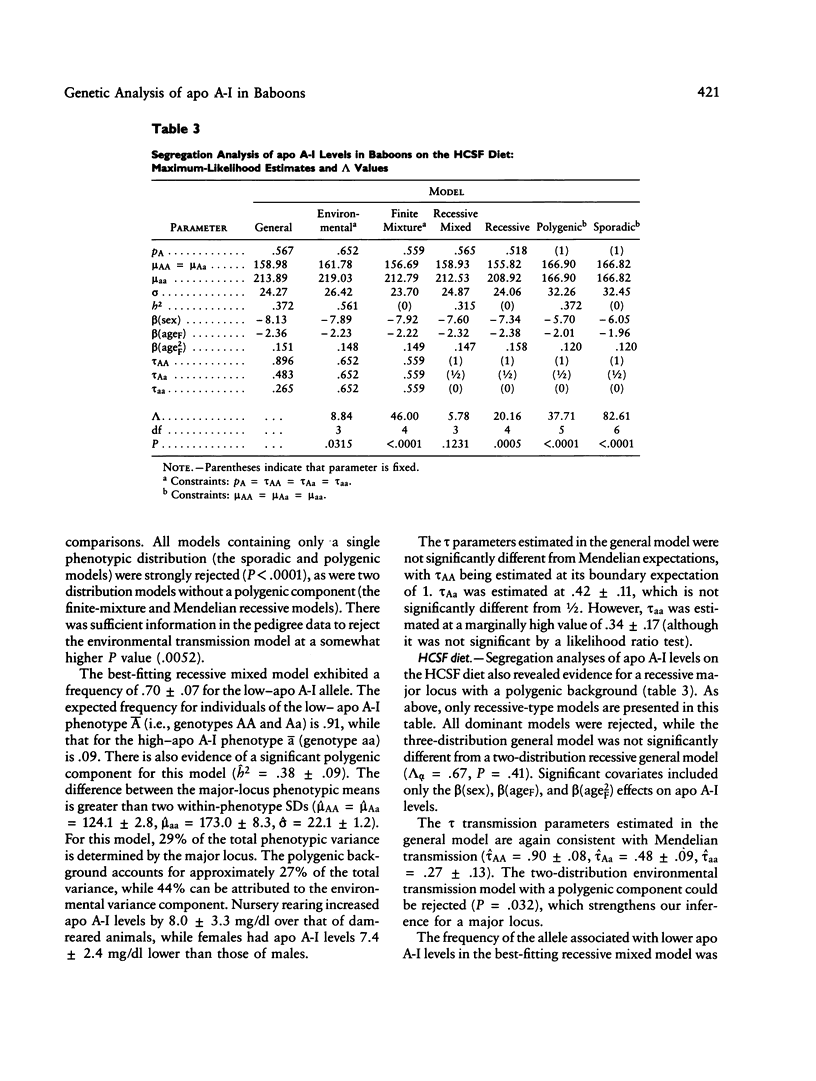
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos C. I., Elston R. C., Srinivasan S. R., Wilson A. F., Cresanta J. L., Ward L. J., Berenson G. S. Linkage and segregation analyses of apolipoproteins A1 and B, and lipoprotein cholesterol levels in a large pedigree with excess coronary heart disease: the Bogalusa Heart Study. Genet Epidemiol. 1987;4(2):115–128. doi: 10.1002/gepi.1370040206. [DOI] [PubMed] [Google Scholar]
- Avogaro P., Bon G. B., Cazzolato G., Quinci G. B. Are apolipoproteins better discriminators than lipids for atherosclerosis? Lancet. 1979 Apr 28;1(8122):901–903. doi: 10.1016/s0140-6736(79)91375-8. [DOI] [PubMed] [Google Scholar]
- Berg K., Kondo I., Drayna D., Lawn R. "Variability gene" effect of cholesteryl ester transfer protein (CETP) genes. Clin Genet. 1989 Jun;35(6):437–445. doi: 10.1111/j.1399-0004.1989.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Berg K. Twin studies of coronary heart disease and its risk factors. Acta Genet Med Gemellol (Roma) 1984;33(3):349–361. doi: 10.1017/s0001566000005808. [DOI] [PubMed] [Google Scholar]
- Boehnke M., Moll P. P., Kottke B. A., Weidman W. H. Partitioning the variability of fasting plasma glucose levels in pedigrees. Genetic and environmental factors. Am J Epidemiol. 1987 Apr;125(4):679–689. doi: 10.1093/oxfordjournals.aje.a114581. [DOI] [PubMed] [Google Scholar]
- Borecki I. B., Laskarzewski P., Rao D. C. Genetic factors influencing apolipoprotein AI and AII levels in a kindred with premature coronary heart disease. Genet Epidemiol. 1988;5(6):393–406. doi: 10.1002/gepi.1370050604. [DOI] [PubMed] [Google Scholar]
- De Backer G., Rosseneu M., Deslypere J. P. Discriminative value of lipids and apoproteins in coronary heart disease. Atherosclerosis. 1982 Apr;42(2-3):197–203. doi: 10.1016/0021-9150(82)90150-2. [DOI] [PubMed] [Google Scholar]
- Elston R. C., Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523–542. doi: 10.1159/000152448. [DOI] [PubMed] [Google Scholar]
- Flow B. L., Cartwright T. C., Kuehl T. J., Mott G. E., Kraemer D. C., Kruski A. W., Williams J. D., McGIll H. C., Jr Genetic effects on serum cholesterol concentrations in baboons. J Hered. 1981 Mar-Apr;72(2):97–103. doi: 10.1093/oxfordjournals.jhered.a109461. [DOI] [PubMed] [Google Scholar]
- Flow B. L., Mott G. E., Kelley J. L. Genetic mediation of lipoprotein cholesterol and apoprotein concentrations in the baboon (Papio sp.). Atherosclerosis. 1982 May;43(1):83–94. doi: 10.1016/0021-9150(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Flow B. L., Mott G. E. Relationship of high density lipoprotein cholesterol to cholesterol metabolism in the baboon (Papio sp.). J Lipid Res. 1984 May;25(5):469–473. [PubMed] [Google Scholar]
- Hamsten A., Iselius L., Dahlén G., de Faire U. Genetic and cultural inheritance of serum lipids, low and high density lipoprotein cholesterol and serum apolipoproteins A-I, A-II and B. Atherosclerosis. 1986 Jun;60(3):199–208. doi: 10.1016/0021-9150(86)90166-8. [DOI] [PubMed] [Google Scholar]
- Hasstedt S. J. A mixed-model likelihood approximation on large pedigrees. Comput Biomed Res. 1982 Jun;15(3):295–307. doi: 10.1016/0010-4809(82)90064-7. [DOI] [PubMed] [Google Scholar]
- Hasstedt S. J., Albers J. J., Cheung M. C., Jorde L. B., Wilson D. E., Edwards C. Q., Cannon W. N., Ash K. O., Williams R. R. The inheritance of high density lipoprotein cholesterol and apolipoproteins A-I and A-II. Atherosclerosis. 1984 Apr;51(1):21–29. doi: 10.1016/0021-9150(84)90141-2. [DOI] [PubMed] [Google Scholar]
- Hopper J. L., Mathews J. D. Extensions to multivariate normal models for pedigree analysis. Ann Hum Genet. 1982 Oct;46(Pt 4):373–383. doi: 10.1111/j.1469-1809.1982.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Kammerer C. M., Mott G. E., Carey K. D., McGill H. C., Jr Effects of selection for serum cholesterol concentrations on serum lipid concentrations and body weight in baboons. Am J Med Genet. 1984 Oct;19(2):333–345. doi: 10.1002/ajmg.1320190216. [DOI] [PubMed] [Google Scholar]
- Kaprio J., Ferrell R. E., Kottke B. A., Sing C. F. Smoking and reverse cholesterol transport: evidence for gene-environment interaction. Clin Genet. 1989 Oct;36(4):266–268. doi: 10.1111/j.1399-0004.1989.tb03201.x. [DOI] [PubMed] [Google Scholar]
- Kondo I., Berg K., Drayna D., Lawn R. DNA polymorphism at the locus for human cholesteryl ester transfer protein (CETP) is associated with high density lipoprotein cholesterol and apolipoprotein levels. Clin Genet. 1989 Jan;35(1):49–56. doi: 10.1111/j.1399-0004.1989.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Kottke B. A., Zinsmeister A. R., Holmes D. R., Jr, Kneller R. W., Hallaway B. J., Mao S. J. Apolipoproteins and coronary artery disease. Mayo Clin Proc. 1986 May;61(5):313–320. doi: 10.1016/s0025-6196(12)61947-8. [DOI] [PubMed] [Google Scholar]
- Kuusi T., Kesäniemi Y. A., Vuoristo M., Miettinen T. A., Koskenvuo M. Inheritance of high density lipoprotein and lipoprotein lipase and hepatic lipase activity. Arteriosclerosis. 1987 Jul-Aug;7(4):421–425. doi: 10.1161/01.atv.7.4.421. [DOI] [PubMed] [Google Scholar]
- Lalouel J. M., Rao D. C., Morton N. E., Elston R. C. A unified model for complex segregation analysis. Am J Hum Genet. 1983 Sep;35(5):816–826. [PMC free article] [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- LeBoeuf R. C., Doolittle M. H., Montcalm A., Martin D. C., Reue K., Lusis A. J. Phenotypic characterization of the Ath-1 gene controlling high density lipoprotein levels and susceptibility to atherosclerosis. J Lipid Res. 1990 Jan;31(1):91–101. [PubMed] [Google Scholar]
- Lewis D. S., Mott G. E., McMahan C. A., Masoro E. J., Carey K. D., McGill H. C., Jr Deferred effects of preweaning diet on atherosclerosis in adolescent baboons. Arteriosclerosis. 1988 May-Jun;8(3):274–280. doi: 10.1161/01.atv.8.3.274. [DOI] [PubMed] [Google Scholar]
- Lewontin R C. The Interaction of Selection and Linkage. I. General Considerations; Heterotic Models. Genetics. 1964 Jan;49(1):49–67. doi: 10.1093/genetics/49.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCluer J. W., Kammerer C. M., Blangero J., Dyke B., Mott G. E., VandeBerg J. L., McGill H. C., Jr Pedigree analysis of HDL cholesterol concentration in baboons on two diets. Am J Hum Genet. 1988 Oct;43(4):401–413. [PMC free article] [PubMed] [Google Scholar]
- Maciejko J. J., Holmes D. R., Kottke B. A., Zinsmeister A. R., Dinh D. M., Mao S. J. Apolipoprotein A-I as a marker of angiographically assessed coronary-artery disease. N Engl J Med. 1983 Aug 18;309(7):385–389. doi: 10.1056/NEJM198308183090701. [DOI] [PubMed] [Google Scholar]
- McGill H. C., Jr, McMahan C. A., Kruski A. W., Mott G. E. Relationship of lipoprotein cholesterol concentrations to experimental atherosclerosis in baboons. Arteriosclerosis. 1981 Jan-Feb;1(1):3–12. doi: 10.1161/01.atv.1.1.3. [DOI] [PubMed] [Google Scholar]
- Moll P. P., Michels V. V., Weidman W. H., Kottke B. A. Genetic determination of plasma apolipoprotein AI in a population-based sample. Am J Hum Genet. 1989 Jan;44(1):124–139. [PMC free article] [PubMed] [Google Scholar]
- Moll P. P., Sing C. F., Williams R. R., Mao S. J., Kottke B. A. The genetic determination of plasma apolipoprotein A-I levels measured by radioimmunoassay: a study of high-risk pedigrees. Am J Hum Genet. 1986 Mar;38(3):361–372. [PMC free article] [PubMed] [Google Scholar]
- Mott G. E., Jackson E. M., McMahan C. A., McGill H. C., Jr Cholesterol metabolism in adult baboons is influenced by infant diet. J Nutr. 1990 Mar;120(3):243–251. doi: 10.1093/jn/120.3.243. [DOI] [PubMed] [Google Scholar]
- Mott G. E., McMahan C. A., Kelley J. L., Farley C. M., McGill H. C., Jr Influence of infant and juvenile diets on serum cholesterol, lipoprotein cholesterol, and apolipoprotein concentrations in juvenile baboons (Papio sp.). Atherosclerosis. 1982 Nov;45(2):191–202. doi: 10.1016/0021-9150(82)90138-1. [DOI] [PubMed] [Google Scholar]
- Mott G. E., McMahan C. A., McGill H. C., Jr Diet and sire effects on serum cholesterol and cholesterol absorption in infant baboons (Papio cynocephalus). Circ Res. 1978 Sep;43(3):364–371. doi: 10.1161/01.res.43.3.364. [DOI] [PubMed] [Google Scholar]
- Paigen B., Mitchell D., Reue K., Morrow A., Lusis A. J., LeBoeuf R. C. Ath-1, a gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3763–3767. doi: 10.1073/pnas.84.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paigen B., Nesbitt M. N., Mitchell D., Albee D., LeBoeuf R. C. Ath-2, a second gene determining atherosclerosis susceptibility and high density lipoprotein levels in mice. Genetics. 1989 May;122(1):163–168. doi: 10.1093/genetics/122.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. B., Wasserman A. G., Muesing R. A., Schlesselman S. E., Larosa J. C., Ross A. M. Lipoprotein and apolipoprotein levels in angiographically defined coronary atherosclerosis. Am J Cardiol. 1985 Jun 1;55(13 Pt 1):1459–1462. doi: 10.1016/0002-9149(85)90953-1. [DOI] [PubMed] [Google Scholar]
- Sistonen P., Ehnholm C. On the heritability of serum high density lipoprotein in twins. Am J Hum Genet. 1980 Jan;32(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Williams M. C., Kushwaha R. S., McGill H. C., Jr Quantitation of baboon lipoproteins by high performance gel exclusion chromatography. Lipids. 1987 May;22(5):366–374. doi: 10.1007/BF02534008. [DOI] [PubMed] [Google Scholar]


