Abstract
Macrophage-derived chemokine (MDC) has been reported to inhibit different HIV-1 strains in activated peripheral blood mononuclear cells (T cell blasts), although other investigators have not confirmed these findings. Here we demonstrate that MDC inhibits the replication of CCR5-dependent (R5) HIV-1BaL in monocyte-derived macrophages (MDM), but not in T cell blasts, although with variable potency depending on donor variability. Analysis of HIV-1BaL proviral DNA synthesis in MDM indicated that the suppressive effect of MDC did not involve inhibition of early events such as entry or reverse transcription. Finally, an inverse correlation was observed between the levels of endogenous MDC secreted by uninfected MDM of different donors and the efficiency of different HIV strains, including two primary isolates with different coreceptor usage, to replicate in these cells. Thus, MDC represents an example of a chemokine inhibiting HIV replication in macrophages acting at one or more postentry levels in the virus life cycle.
Replication of HIV in vivo primarily occurs in CD4+ T lymphocytes and mononuclear phagocytes (1), two fundamental target cells also infectable in vitro. In this regard, T cell tropic [also known as “syncytium-inducing” (SI) viruses] vs. macrophage tropic/non-SI HIV isolates have been described (2). Chemokines, such as regulated upon activation normal T cell expressed and secreted (RANTES), macrophage inflammatory protein (MIP)-1α, and MIP-1β have shown inhibitory effects on the replication of macrophage tropic/non-SI viruses in T cells (3) and, in some studies, also in monocytes and/or monocyte-derived macrophages (MDM) (4, 5). Their antiviral effect has been elegantly demonstrated to reside in the binding to CCR5, a cell surface receptor also serving as viral coreceptor for entry into CD4+ cells (6). Furthermore, CXCR4 serves as coreceptor for SI HIV-1 strains (7, 8), whereas its ligand stromal cell-derived factor-1 can selectively interfere with replication of these viruses. In addition to CCR5 and CXCR4, other chemokine receptors, including CCR2B, CCR3, and CCR8 can act as viral coreceptors, although rarely, and their ligands [including monocyte chemotactic protein (MCP) 2, MCP-3, eotaxin, thymus- and activation- regulated chemokine (TARC), and I-309] have demonstrated inhibitory effects on viral replication (9–12).
Macrophage-derived chemokine (MDC) is produced by several cell types, including macrophages, dendritic cells, and B and T lymphocytes (13–17), and can induce chemotaxis of monocytes, T lymphocytes, natural killer cells, and, in particular, dendritic cells (17, 18). A truncated form of MDC, named native MDC, lacking the first two N-terminus amino acids (19), originally was purified from the supernatant of immortalized CD8+ T cells of HIV seropositive individuals and shown to exert a broad spectrum inhibitory activity against different HIV and simian immunodeficiency virus strains regardless of their differential usage of chemokine entry coreceptors (19). This finding was, however, confuted by some (20, 21), but confirmed by other investigators who focused on HIV replication in activated T cells (22). The antiviral mechanism of action of MDC in activated T cells remains undefined (19, 22). In addition, whether MDC can inhibit HIV replication in macrophages has not been addressed, although lack of interference with viral entry in these cells by a surrogate β-galactosidase-reporter infection system has been mentioned (20).
In the present study, we have investigated whether full-length synthetic MDC could interfere with HIV replication in either activated primary T cells or in MDM of seronegative individuals. Our results indicate that MDC exerts a suppressive effect on HIV replication in MDM, but not in activated T cells by an entry-independent mechanism. Furthermore, the differential ability of HIV to replicate in MDM of different donors was found inversely correlated to the constitutive capacity of these cells to secrete MDC.
Materials and Methods
T Cell Blasts.
Peripheral blood mononuclear cells of healthy seronegative individuals were stimulated for 72 h with 5 μg/ml of phytohemagglutinin (PHA-P, Sigma), washed extensively, and resuspended in RPMI 1640 (BioWhittaker) containing 10% FCS (HyClone) and recombinant IL-2 (Boehringer Mannheim) (complete medium). In some experiments, peripheral blood mononuclear cells were depleted of CD8+ cells (mostly T cells) by a single round of immunoconjugated beads following the manufacturer's instructions (Dynatech, Dynal, Oslo).
MDM.
Monocytes were isolated from peripheral blood mononuclear cells obtained from buffy coats of healthy HIV seronegative blood donors by Ficoll-Hypaque silica gradient (Amersham Pharmacia), followed by sedimentation onto an isoosmotic Percoll gradient (Amersham Pharmacia), as described (23). Purity was consistently ≥ 90%, as determined by FACS analysis of CD14 expression (23). Monocytes were seeded in 48-well plastic plates (Falcon, Becton Dickinson Labware) at 2–3 × 105 cells/ml in DMEM (BioWhittaker) supplemented with 10% FCS (HyClone) and 5% pooled human serum. Media and sera were monitored for low content of endotoxin by the Limulus amoebocyte lysate assay (BioWhittaker).
HIV Infections.
Three-day stimulated T cell blasts were washed and resuspended in complete medium before infection with either the macrophage-tropic R5 HIV-1BaL or the T lymphotropic X4 HIV-1LAI/IIIB strains (24) at the multiplicity of infection (moi) of 0.1. Five- to 7-day-old MDM were infected with HIV-1BaL at the same m.o.i. Fifty percent of culture media were replaced with fresh media twice a week. Aliquots of culture supernatants were harvested every 2–3 days and stored at −80°C. Cell pellets were prepared at different time points and stored at −80°C. HIV replication was monitored by a Mg2+-dependent reverse transcription (RT) activity assay on culture supernatants (25).
HIV DNA Quantitation by TaqMan.
The kinetics and levels of HIV DNA accumulation in MDM were determined by the TaqMan assay with an ABI 7700 Prism instrument (Perkin–Elmer Applied Biosystems), as recently described in detail (25). Briefly, DNase-treated HIV-1BaL was added to MDM cultures at the multiplicity of infection of 0.1. Cells then were harvested at different times after infection, washed extensively, counted, and centrifuged at 3,000 rpm for 10 min. Cell pellets then were lysed in a buffer containing proteinase K, and the DNA was extracted by phenol-chloroform and precipitated by ethanol. DNA preparations corresponding to 105 cells were amplified with the following primers: forward, 5′-ACA TCA AGC AGC CAT GCA AAT-3′ (1368 to 1388); reverse, 5′-ATC TGG CCT GGT GCA ATA GG-3′ (1472 to 1453); and probe (FAM), 5′-CAT CAA TGA GGA AGC TGCAGA ATG GGA TAG A-3′ (TAMRA) (1401 to 1431); the number in parentheses indicated the primer's position in the HIV-1HXB2 molecular clone (26). The thermal cycling conditions were 50°C for 2 min, 95°C for 12 min, and 40 cycles of 95°C for 15 s and 65°C for 1 min. Each run contained standards consisting of DNA obtained from serial dilutions of the chronically infected ACH-2 T cells, which contain one integrated copy of infectious proviral DNA per cell. As reported (25), a linear distribution (r = 0.99) was obtained between 2 and 31,250 ACH-2 T cells. Curve fitting and interpolation of unknown values was performed by using the taqman software. Separate β-actin amplifications were carried out for control by using a commercial kit (Perkin–Elmer) following the manufacturer's instructions.
Functional Studies.
MDM migration was evaluated by using a chemotaxis microchamber technique with 5 μm polycarbonate filters (Neuroprobe, Cabin John, MD), as described (18). Changes in intracellular calcium concentration were monitored by the fluorescent probe Fura-2 (Calbiochem) by using a LS-5B fluorimeter (Perkin–Elmer) (22, 25).
Source of MDC.
Synthetic MDC was obtained by ICOS (kind gift of Pat Gray) after analysis by HPLC, gel electrophoresis, and N-terminal sequencing. Binding studies suggest that full-length MDC binds CCR4 with high affinity (IC50: 30 pM). MDC and MCP-1 were tested by ELISA as reported (14, 27).
Results
MDC Inhibits HIV-1BaL Replication in MDM but Not in T Cell Blasts.
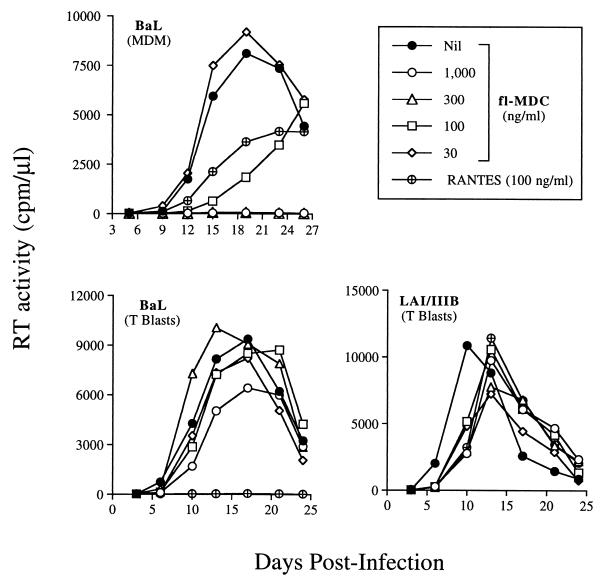
Synthetic lipopolysaccharide-free (by the limulus amoebocyte cell lysate test) MDC inhibited the replication of the R5 HIV-1BaL in MDM cultures established from eight of 10 donors, whereas it did not show significant effect on virus production in the remainders. MDC inhibition of HIV replication in MDM was observed in a range between 100 and 1,000 ng/ml; in some donors, concentrations of MDC above 100 ng/ml resulted in a near complete inhibition of virus replication (Fig. 1 Upper). In contrast, no substantial modulatory effects on virus replication were observed when MDC was tested on primary T cell blasts infected with HIV-1BaL, that, otherwise, were completely inhibited by RANTES (Fig. 1 Lower Left). RANTES, on the other hand, showed only partial inhibitory effects on HIV-1BaL replication in MDM of some donors (Fig. 1 Upper) and did not inhibit virus production with cell cultures established from other seronegative individuals, as reported (28). Neither MDC nor RANTES significantly inhibited CXCR4-dependent viral replication in T cell blasts, consistently with previous observation (3) (Fig. 1 Lower Right). Because HIV-1LAI/IIIB replication in MDM was only rarely and inconsistently observed, the effect of chemokines on this virus infection was not investigated.
Figure 1.
Selective inhibitory effects of MDC on HIV-1 replication in MDM, but not in primary T cell blasts. (Upper) Concentration-dependent inhibition of R5 HIV-1BaL replication in MDM. Complete suppression by MDC is observed with MDC concentrations equal to or above 300 ng/ml. RANTES shows inhibitory effects comparable to those observed with the same concentration of MDC. (Lower Left) RANTES (300 ng/ml), but not MDC, suppresses HIV-1BaL replication in T cell blasts. MDC was ineffective against virus replication in T cells also when tested at concentrations up to 10 μg/ml. (Lower Right) Lack of effect of MDC or RANTES on HIV-1LAI/IIIB replication in T cell blasts. Inhibition of viral replication also was observed in CD8-depleted T cell blasts, primary CD4+ T cell clones and CB lines either unpolarized or differentiated into Th1 and Th2 cells and infected with either HIV-1BaL or HIV-1LAI/IIIB.
As observed with activated T cell blasts, human cord blood (CB) mononuclear cells differentiated along the T helper (Th) 1 or Th2 pathways in the presence of polarizing signals [such as IL-4 and anti-IL-12 mAbs, for Th2 development and IL-12 plus anti-IL-4 mAb for Th1 differentiation (29)], were infected with HIV-1BaL. Comparable levels of virus replication were observed in these cell lines as recently reported (25), which were substantially inhibited by RANTES, whereas MDC did not interfere with HIV-1 replication in these polarized CB-derived cells or in activated unpolarized (Th0) CB cells (data not shown).
Differences Between MDC Preparations.
The mature form of MDC was made by direct chemical synthesis as described (18) and by recombinant expression in mammalian cells and in yeast. This material was biologically active in terms of its capacity to elicit both Ca2+ fluxes and chemotaxis in cells expressing CCR4 but not other chemokine receptors (30). In addition, these MDC preparations were analyzed by using the following biochemical assays: N-terminal amino acid sequencing, mass spectrometry, analysis of disulfide pairing, and recognition by conformation dependent mAbs. UV spectral analysis also was performed, and the spectra obtained from native MDC were compared with MDC that had been rendered biologically inactive by denaturation. Material obtained from one commercial source that had no biological activity in chemotaxis assays had a UV spectra that was identical to that of denatured rather than native (active) MDC (D. Chantry, personal communication).
We originally had tested different commercially available sources of MDC. Results were inconsistent, with inhibition observed in 5/13 infections (not shown). Because these commercial preparations were not systematically checked by using the above criteria, the reason for these inconsistent results cannot be defined with certainty, but it is tempting to speculate that they relate to the quality of the material. All together, in the MDM cultures established from 13 independent donors in which MDC inhibited HIV-1BaL replication, its activity ranged between 49% and 99%, with a mean inhibition of peak of RT activity production (± SD) of 82.3% (±18.4%).
MDC Does Not Inhibit Entry and RT of HIV-1BaL in MDM.
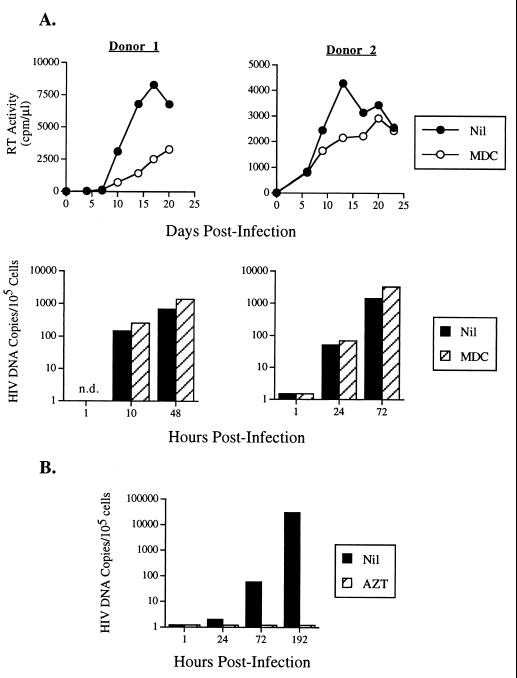
To elucidate whether MDC interfered with entry of HIV-1 into MDM, the kinetics of accumulation of HIV proviral DNA were quantified by real-time PCR using an Applied Biosystems Prism 7700 apparatus (25). In contrast to its inhibitory effects on RT activity production, MDC did not substantial affect HIV DNA accumulation up to 72 h postinfection (Fig. 2A), whereas complete inhibition was observed in MDM incubated with 3′-azido-3′-deoxythymidine (AZT) (Fig. 2B) (31). In some experiments, HIV DNA was evaluated up to 8 days postinfection. Although beyond the linear detection range on the assay, no substantial differences were observed in terms of accumulation of HIV DNA in MDM treated with MDC in comparison to untreated MDM (data not shown). In consideration of the fact that a single round of HIV replication occurs in approximately 24 h in T cells and up to 48 h in macrophages (32), these findings suggest that MDC can interfere with HIV replication in macrophages by acting at a postentry step of the virus life cycle.
Figure 2.
Analysis of HIV DNA accumulation in HIV-1BaL infected MDM in the presence or absence of MDC (240 ng/ml) (A) or 3′-azido3′-deoxythimidine (AZT) (10 μM) (B). In contrast to the inhibitory effects exerted by MDC on HIV replication in MDM of two independent donors, as determined by supernatant-associated RT activity, a faithful indicator of virion production (45), no substantial effects were observed in terms of accumulation of HIV DNA up to 72 h postinfection, as quantified by a TaqMan assay (Applied Biosystems) (25). n.d., not done.
MDC Does Not Deactivate MDM Functions.
We next investigated whether the observed inhibitory effects exerted by MDC on HIV-1 replication could be accounted for by a broad inhibitory effect on macrophage function rather than a selective interference with virus multiplication. MDC (100 ng/ml) induced MDM chemotaxis (with 38 ± 4 vs. 45 ± 3 vs. 21 ± 4 cells migrating in response to MDC vs. fMet-Leu-Phe vs. unstimulated condition, respectively), and secretion of MCP-1 (with 31 ± 2.4 vs. 10 ± 0.8 ng/ml for MDC-stimulated and unstimulated cells, respectively) from these cells (data not shown). Finally, Ca2+ fluxes were determined in response to fMet-Leu-Phe in MDM that had been either prestimulated with MDC or left unstimulated. No evidence of desensitization in terms of Ca2+ fluxes induced by the stimulus was obtained (data not shown). These results indicate that MDC does not cause deactivation of macrophage functions.
Constitutive MDC Secretion and HIV Replication in MDM.
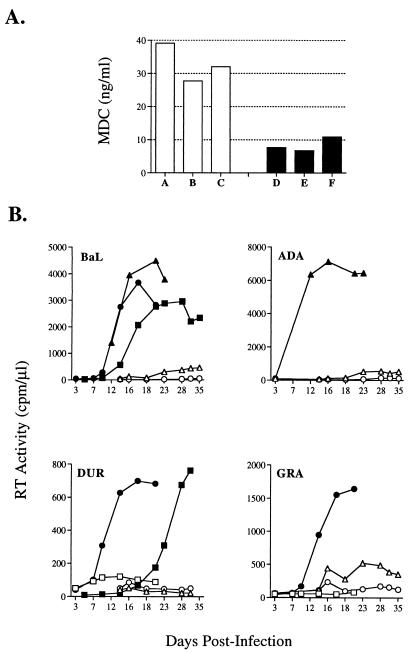
To investigate whether endogenous MDC secretion could influence the ability of HIV-1BaL to replicate in MDM, the levels of MDC were measured at different time points in both uninfected and HIV-infected cultures by ELISA. In agreement with recent reports (14, 16), MDC was detected in the supernatants of all MDM cultures tested (Fig. 3A). Of interest, we observed that HIV-1BaL replication occurred more efficiently in MDM cultures characterized by peak constitutive MDC secretion lower than 15 ng/ml in uninfected parallel cultures, whereas substantially less efficient viral replication was observed when MDM constitutively secreted higher levels of the chemokine (Fig. 3B). This association was further confirmed with infection of MDM by viruses other than HIV-1BaL, and including the laboratory-adapted strain ADA (reported to use CCR3 and CCR8 in addition to CCR5) (9, 11) and two primary isolates: the R5 monotropic GRA and the R5/X4 dual-tropic DUR (27) (Fig. 3B). The levels of MDC secreted at different time points during infection were not decreased in comparison to those of parallel uninfected cultures (data not shown). The possibility that the observed patterns could be explained or contributed by the constitutive ability of MDM to secrete other HIV-regulatory CC chemokines was investigated in the same cell culture supernatants. As reported (33–36) very low levels of RANTES, MIP-1α, and MIP-1β were detected in the uninfected MDM cultures, whereas substantial levels of MCP-1 were observed, as described (27, 35) (Table 1). Thus, the intrinsic ability of macrophages of secreting variable amounts of MDC may represent an important variable in their capacity to support HIV replication once infected.
Figure 3.
Endogenous secretion of MDC from uninfected MDM and HIV replication. MDM culture supernatants from different donors were collected at day 5 and tested for their contents of MDC before infection with HIV-1BaL (A). In some experiments, infections by ADA (B) and by two primary HIV isolates (DUR, R5, and GRA, R5/X4) also were performed. A strong inverse association between levels of endogenously secreted MDC (low: <15 ng/ml, full symbols; high: >15 ng/ml, open symbols) and HIV replicative ability in these MDM cultures was observed.
Table 1.
Chemokine secretion by uninfected MDM of independent donors
| Donor | MDC | MCP-1 | RANTES | MIP-1α | MIP-1β |
|---|---|---|---|---|---|
| A | 39 | 31 | 0.06 | 0.15 | 0.08 |
| B | 28 | 23 | 0.05 | 0.09 | 0.07 |
| C | 32 | 31 | 0.05 | 0.18 | 0.6 |
| D | 7 | ND | ND | ND | ND |
| E | 7.5 | 67 | 0.07 | 0.14 | 0.4 |
| F | 11 | 14 | 0.06 | 0.07 | 0.01 |
The reported values indicate the peak chemokine levels (ng/ml) detected in the supernatants of uninfected MDM cultures. ND, not determined.
Discussion
Our study shows that MDC is an effective inhibitor of R5 HIV-1 replication in MDM acting at one or more postentry steps in the virus life cycle. In agreement with previous observations (20, 21), MDC did not inhibit either R5 or X4 viral replication in activated T lymphocytes, including T cell blasts, Th0, Th1, and Th2 cells differentiated from human CB. Therefore, MDC shows a more restricted spectrum of antiviral activity to that originally ascribed to native MDC (19). Of note, differences in the amino acid sequence at the N terminus were shown to exist between native MDC and MDC (19). Truncated MDC lost binding to CCR4, the only characterized receptor for this chemokine (30), although it maintained chemotactic activity likely via interaction with an undefined cell surface molecule (22).
The lack of effect of MDC on HIV replication in T lymphocytes cannot be explained by lack of CCR4 expression in that all of the cells tested are capable of responding to similar concentrations of MDC (18). In addition, preferential surface expression of CCR4 recently has been shown on Th2 rather than Th1 cells (37–39), although HIV-1 infection of neither cell type was inhibited by MDC.
We also have observed major differences by using independent sources of synthetic MDC, which may in part explain the existing discrepant results reported in the literature. Of note is the fact that enhancement of viral replication, as well as no effects and inhibition, was observed by using a second source of the chemokine. In this regard, enhancement of HIV replication in MDM by both full-length and truncated MDC has been reported (40) by using a commercial source of the chemokine. We believe that in the future it will be important to specify whether the different synthetic MDC had been checked for their folding patterns before biological testing, in that different and often opposite results can be obtained. It is conceivable that an improperly folded molecule may retain some functional properties (i.e., receptor binding and chemotaxis) but may act as an antagonist for other functions, including modulation of HIV replication.
The analysis of HIV DNA accumulation, measured by a quantitative assay (25), has indicated that MDC did not interfere with either entry or RT. Therefore, in contrast to CCR5- or CXCR4-binding chemokines (6), MDC is likely to act at one or more postentry levels in MDM, as previously shown for some anti-inflammatory cytokines, including IL-4, IL-10, and transforming growth factor β in monocytic cells (reviewed in ref. 41).
The observation that relatively high levels of MDC constitutively secreted by MDM are inversely correlated to the ability of HIV to replicate in these cells suggests that endogenous MDC may represent an important determinant of the replicative capacity of the virus in macrophages. In this regard, it has been previously shown that either CD4+ or CD8+ T lymphocytes of some highly exposed, but uninfected, individuals were resistant to infection by R5 viruses as a result of their ability to secrete high levels of CCR5-interacting chemokines (42, 43) or because of anti-CCR5 antibodies (44). Our findings suggest that MDC can play a similar role in the case of macrophage infection, at least in vitro.
The postentry inhibitory capacity of MDC on HIV replication in macrophages suggests that synergistic interactions between chemokines and/or cytokines acting at different levels of the HIV life cycle can occur and represent important determinants of the ultimate capacity of the virus to spread in infected individuals.
Note. After the completion of this work, Alfredo Garzino-Demo et al. from the Institute of Human Virology, Baltimore, MD, independently observed inhibition of HIV replication in monocyte-derived macrophages by a commercial source of MDC (Peprotech). Their preliminary analyses suggest that such an inhibitory effect of MDC occurs at a postentry level of the HIV life cycle, as indicated in the present study.
Acknowledgments
We thank David Chantry and Pat Gray from ICOS Corporation for provision of quality-controlled MDC and helpful suggestions, Alessia Verani for testing the potential presence of contaminant lipopolysaccharide, and Andrea Brambilla for assistance with the real-time PCR assay for quantitation of HIV DNA. This work was supported in part by grants of the National Project for Research Against AIDS of the Istituto Superiore di Sanità, Rome. M.M. is a fellow of the Associazione Nazionale per la Lotta contro l'AIDS.
Abbreviations
- CB
cord blood
- MDM
monocyte-derived macrophages
- MDC
macrophage-derived chemokine
- RANTES
regulated upon activation normal T cell expressed and secreted
- MIP
macrophage inflammatory protein
- MCP
monocyte chemotactic protein
- RT
reverse transcription
- Th
T helper
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160359197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160359197
References
- 1.Fauci A S. Nature (London) 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 2.Koot M, Vos A H, Keet R P, de Goede R E, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 4.Capobianchi M R, Abbate I, Antonelli G, Turriziani O, Dolei A, Dianzani F. AIDS Res Hum Retroviruses. 1998;14:233–240. doi: 10.1089/aid.1998.14.233. [DOI] [PubMed] [Google Scholar]
- 5.Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells T N, et al. J Exp Med. 1998;187:439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Littman D R. Cell. 1998;93:677–680. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 7.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. Nature (London) 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 8.Nagasawa T, Nakajima T, Tachibana K, Iizasa H, Bleul C C, Yoshie O, Matsushima K, Yoshida N, Springer T A, Kishimoto T. Proc Natl Acad Sci USA. 1996;93:14726–14729. doi: 10.1073/pnas.93.25.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, et al. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, et al. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 12.Gong W, Howard O M, Turpin J A, Grimm M C, Ueda H, Gray P W, Raport C J, Oppenheim J J, Wang J M. J Biol Chem. 1998;273:4289–4292. doi: 10.1074/jbc.273.8.4289. [DOI] [PubMed] [Google Scholar]
- 13.Andrew D P, Chang M S, McNinch J, Wathen S T, Rihanek M, Tseng J, Spellberg J P, Elias C G., 3rd J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- 14.Bonecchi R, Sozzani S, Stine J T, Luini W, D'Amico G, Allavena P, Chantry D, Mantovani A. Blood. 1998;92:2668–2671. [PubMed] [Google Scholar]
- 15.Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink A G, Sideras P. J Exp Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodenburg R J, Brinkhuis R F, Peek R, Westphal J R, Van Den Hoogen F H, van Venrooij W J, van de Putte L B. J Leukocyte Biol. 1998;63:606–611. doi: 10.1002/jlb.63.5.606. [DOI] [PubMed] [Google Scholar]
- 17.Tang H L, Cyster J G. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- 18.Godiska R, Chantry D, Raport C J, Sozzani S, Allavena P, Leviten D, Mantovani A, Gray P W. J Exp Med. 1997;185:1595–1604. doi: 10.1084/jem.185.9.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 20.Lee B, Rucker J, Doms R W, Tsang M, Hu X, Dietz M, Bailer R, Montaner L J, Gerard C, Sullivan N, et al. Science. 1998;281:487–487a. [Google Scholar]
- 21.Arenzana-Seisdedos F, Amara A, Thomas D, Virelizier J L. Science. 1998;281:487–487a. [Google Scholar]
- 22.Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, De Clercq E, Schols D, Van Damme J. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 23.Colotta F, Bersani L, Lazzarin A, Poli G, Mantovani A. J Immunol. 1985;134:3524–3531. [PubMed] [Google Scholar]
- 24.Berger E A, Doms R W, Fenyo E M, Korber B T, Littman D R, Moore J P, Sattentau Q J, Schuitemaker H, Sodroski J, Weiss R A. Nature (London) 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 25.Vicenzi E, Bordignon P P, Biswas P, Brambilla A, Bovolenta C, Cota M, Sinigaglia F, Poli G. J Virol. 1999;73:7515–7523. doi: 10.1128/jvi.73.9.7515-7523.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorelick N R J, Henderson L E. Human Retroviruses and AIDS. Los Alamos, NM: Los Alamos National Laboratory; 1994. [Google Scholar]
- 27.Mengozzi M, De Filippi C, Transidico P, Biswas P, Cota M, Ghezzi S, Vicenzi E, Mantovani A, Sozzani S, Poli G. Blood. 1999;93:1851–1857. [PubMed] [Google Scholar]
- 28.Schmidtmayerova H, Sherry B, Bukrinsky M. Nature (London) 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 29.D'Ambrosio D, Cippitelli M, Cocciolo M G, Mazzeo D, Di Lucia P, Lang R, Sinigaglia F, Panina-Bordignon P. J Clin Invest. 1998;101:252–262. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imai T, Chantry D, Raport C J, Wood C L, Nishimura M, Godiska R, Yoshie O, Gray P W. J Biol Chem. 1998;273:1764–1768. doi: 10.1074/jbc.273.3.1764. [DOI] [PubMed] [Google Scholar]
- 31.Poli G, Orenstein J M, Kinter A, Folks T M, Fauci A S. Science. 1989;244:575–577. doi: 10.1126/science.2470148. [DOI] [PubMed] [Google Scholar]
- 32.Kim S Y, Byrn R, Groopman J, Baltimore D. J Virol. 1989;63:3708–3713. doi: 10.1128/jvi.63.9.3708-3713.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmidtmayerova H, Nottet H S, Nuovo G, Raabe T, Flanagan C R, Dubrovsky L, Gendelman H E, Cerami A, Bukrinsky M, Sherry B. Proc Natl Acad Sci USA. 1996;93:700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canque B, Rosenzwajg M, Gey A, Tartour E, Fridman W H, Gluckman J C. Blood. 1996;87:2011–2019. [PubMed] [Google Scholar]
- 35.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, et al. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Swingler S, Mann A, Jacque J, Brichacek B, Sasseville V G, Williams K, Lackner A A, Janoff E N, Wang R, Fisher D, Stevenson M. Nat Med. 1999;5:997–103. doi: 10.1038/12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto F, Lenig D, Mackay C R, Lanzavecchia A. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 39.Bonecchi R, Bianchi G, Bordignon P P, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriuchi H, Moriuchi M. AIDS. 1999;13:994–996. doi: 10.1097/00002030-199905280-00019. [DOI] [PubMed] [Google Scholar]
- 41.Vicenzi E, Biswas P, Mengozzi M, Poli G. J Leukocyte Biol. 1997;62:34–40. doi: 10.1002/jlb.62.1.34. [DOI] [PubMed] [Google Scholar]
- 42.Paxton W A, Martin S R, Tse D, O'Brien T R, Skurnick J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 43.Furci L, Scarlatti G, Burastero S, Tambussi G, Colognesi C, Quillent C, Longhi R, Loverro P, Borgonovo B, Gaffi D, et al. J Exp Med. 1997;186:455–460. doi: 10.1084/jem.186.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopalco L, Barassi C, Pastori C, Longhi R, Burastero S E, Tambussi G, Mazzotta F, Lazzarin A, Clerici M, Siccardi A G. J Immunol. 2000;164:3426–3433. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- 45.Fernie B F, Poli G, Fauci A S. J Virol. 1991;65:3968–3971. doi: 10.1128/jvi.65.7.3968-3971.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]