Abstract
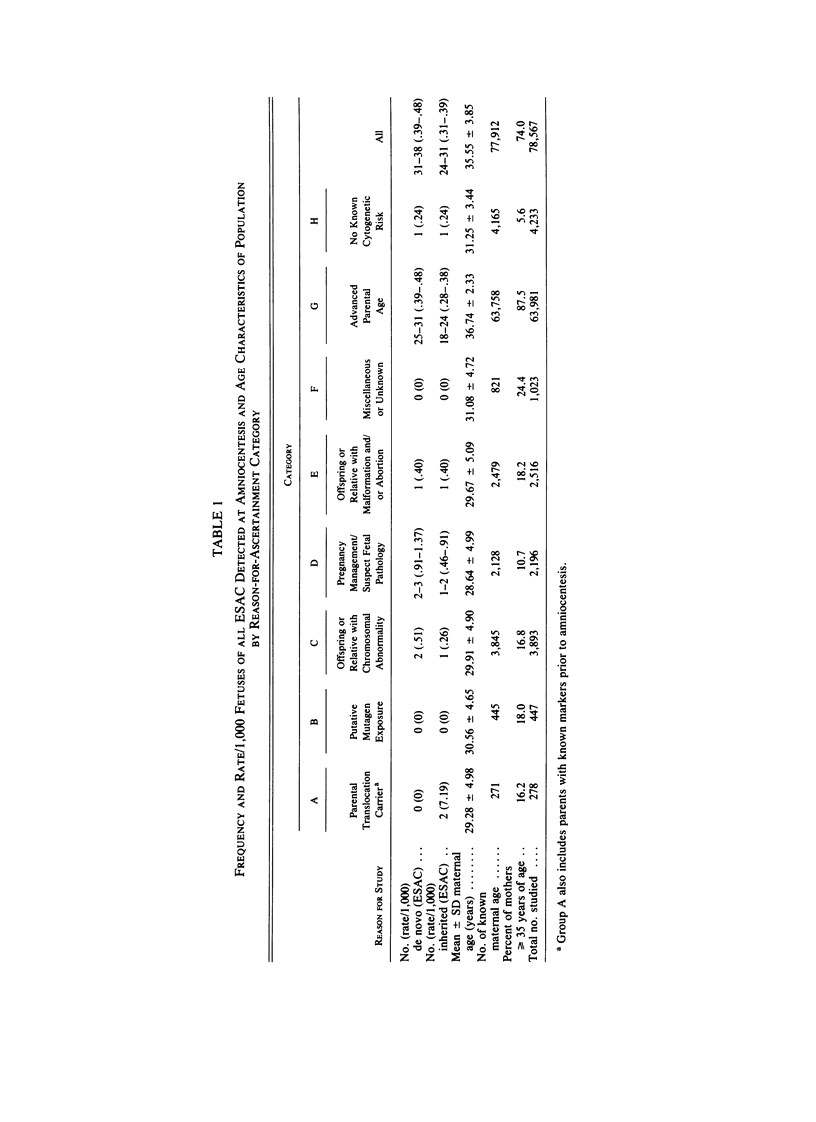
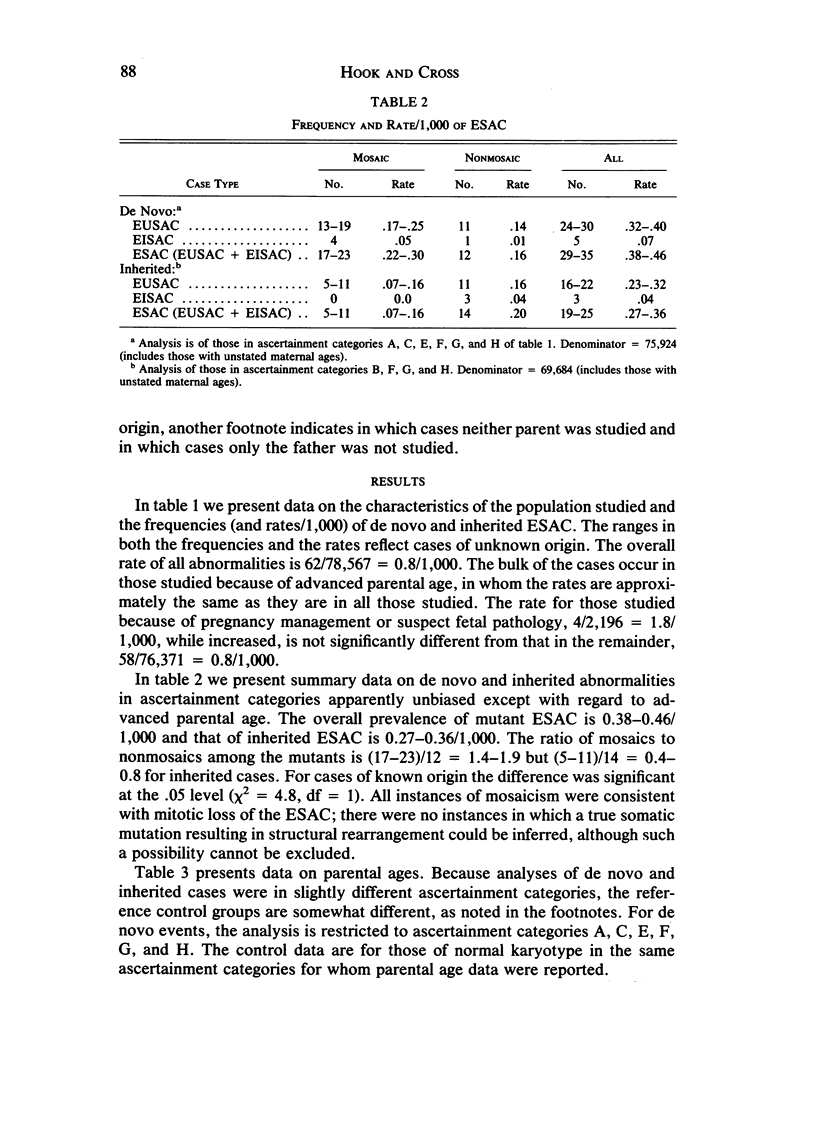
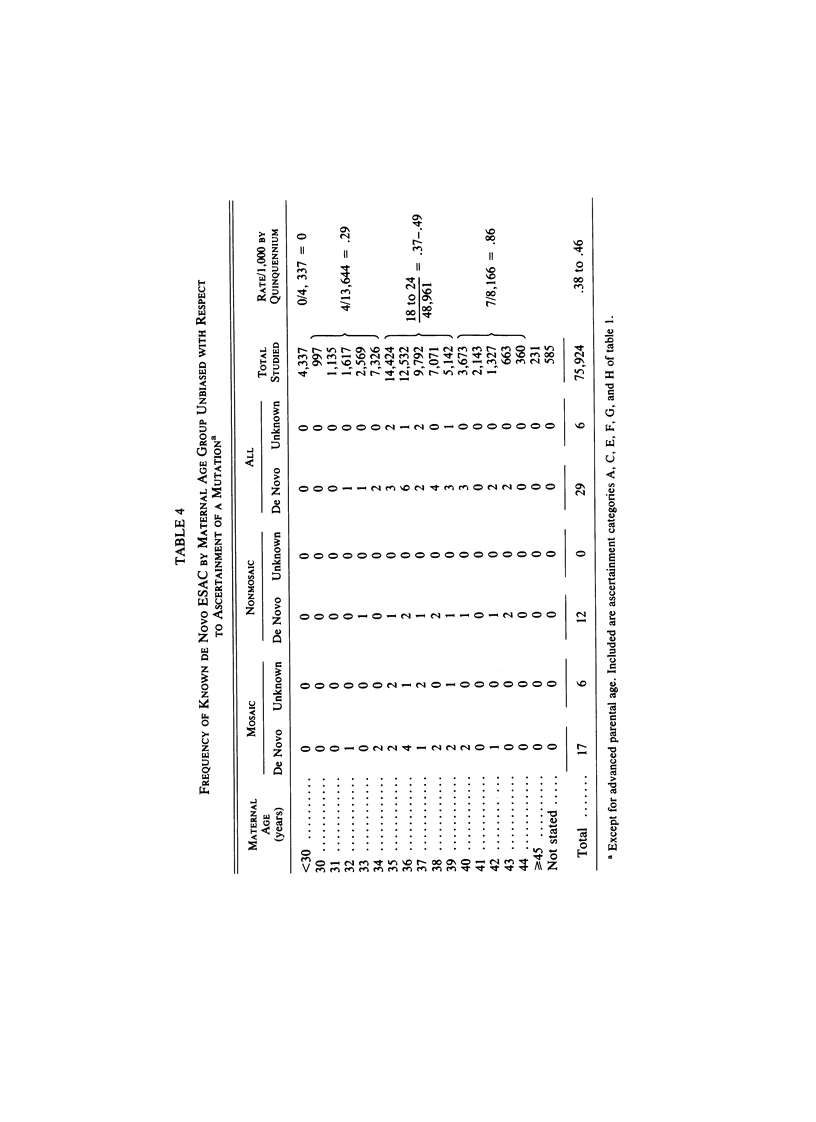
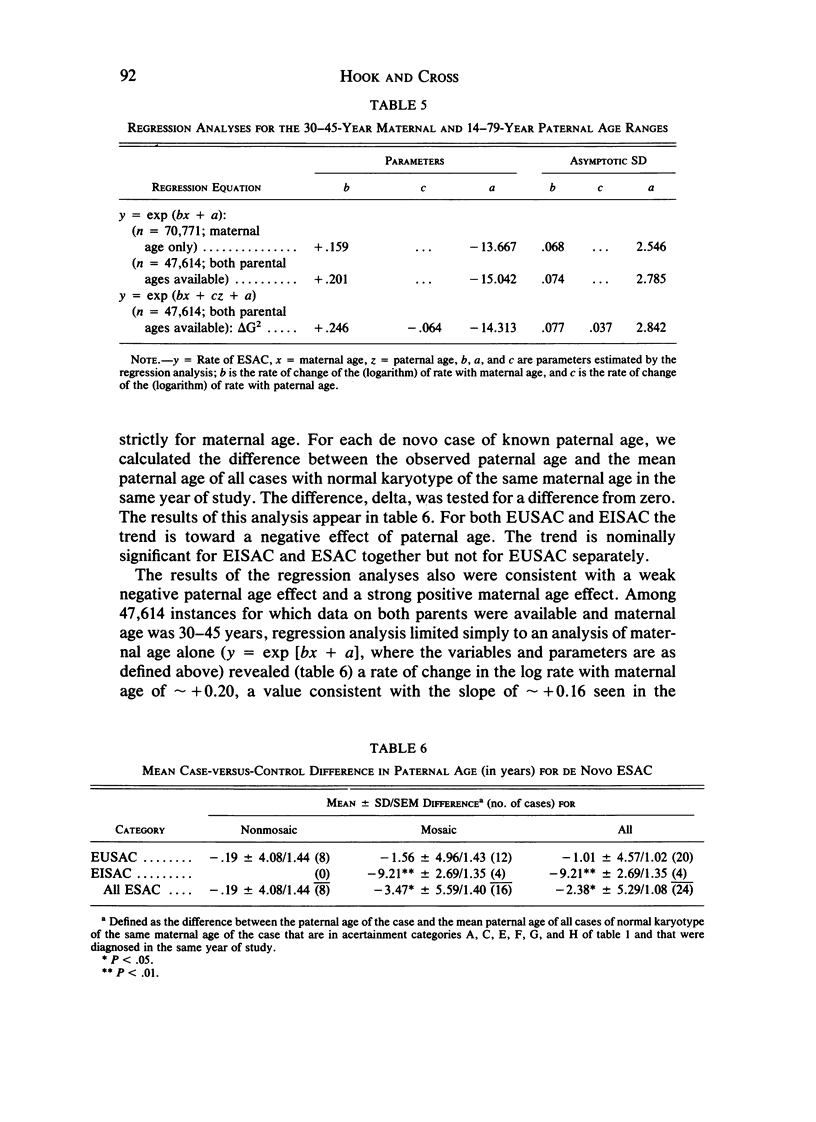
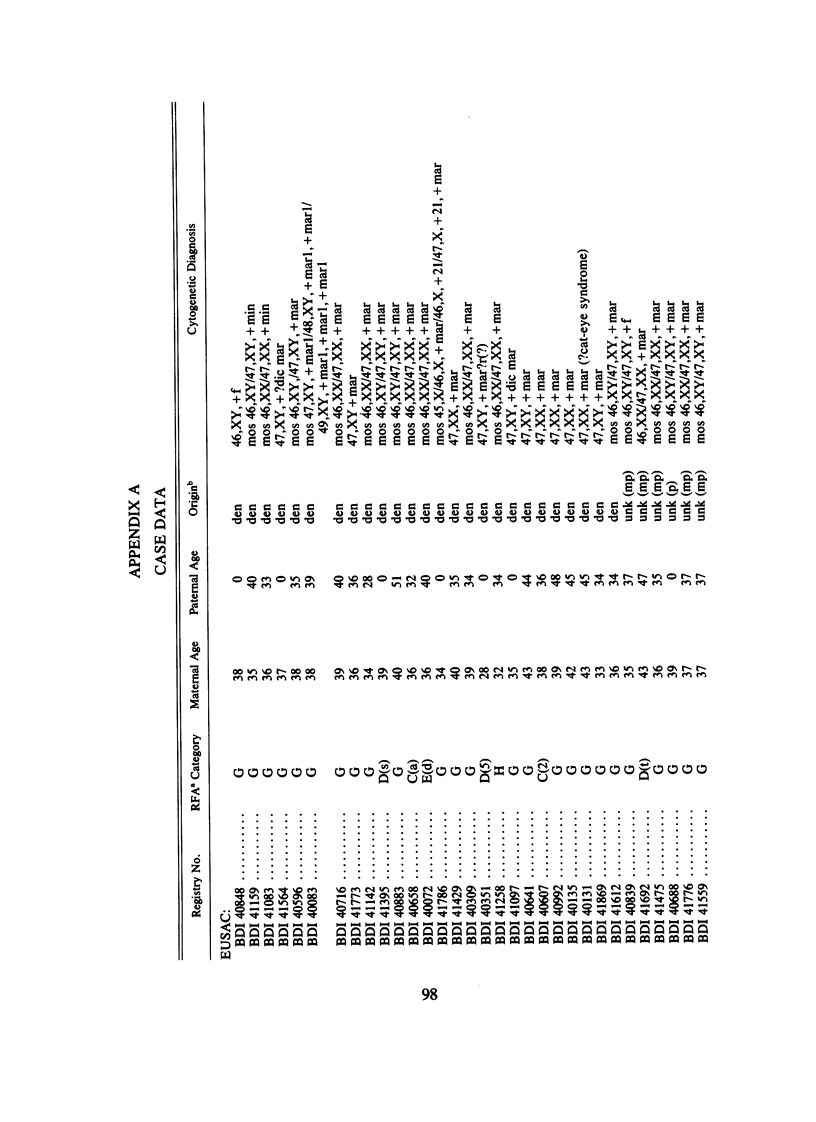
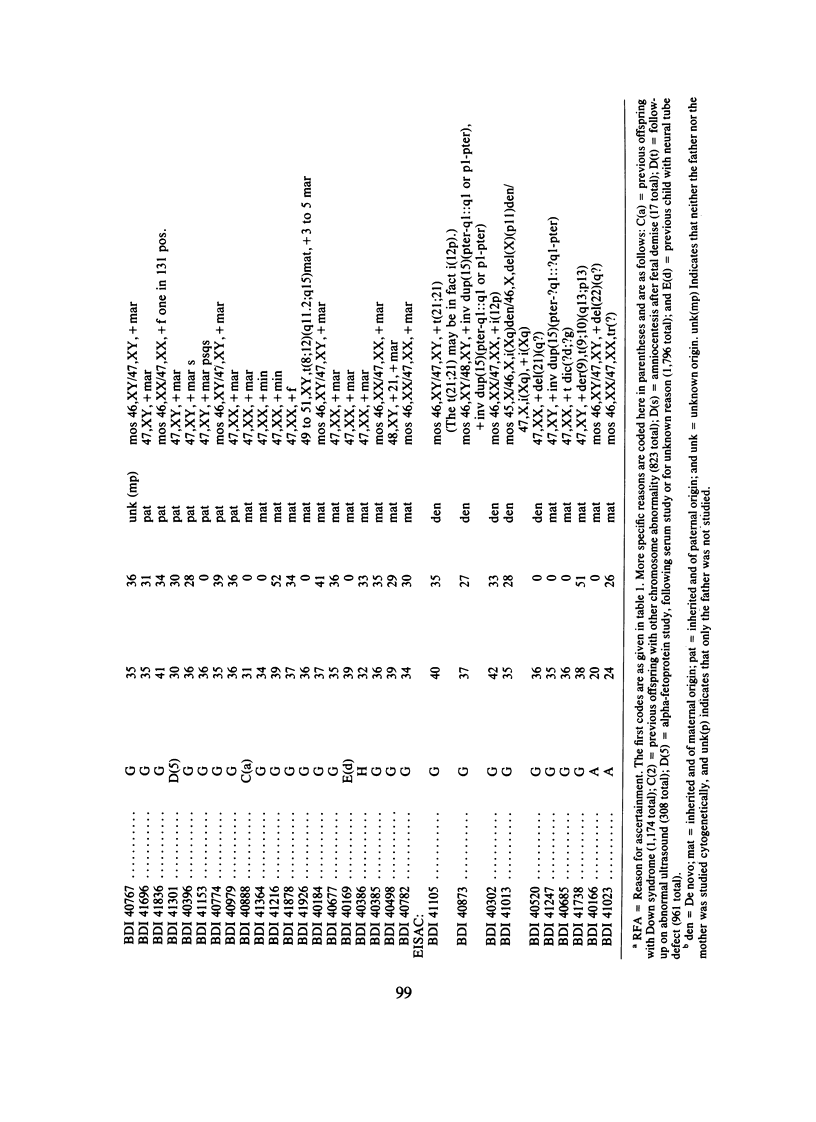
We analyzed rates of extra structurally abnormal chromosomes (ESAC) detected in prenatal cytogenetic diagnoses of amniotic fluid reported to the New York Chromosome Registry. These karyotypes include both extra unidentified structurally abnormal chromosomes (EUSAC)--often denoted as "markers"--and extra identified structurally abnormal chromosomes (EISAC). The rate of all EUSAC was 0.64/1,000 (0.32-0.40/1,000 mutant and 0.23-0.32 inherited), and that of all EISAC was 0.11/1,000 (0.07/1,000 mutant and 0.04/1,000 inherited). The rate of all ESAC was approximately 0.8/1,000-0.4-0.5/1,000 mutant and 0.3-0.4/1,000 inherited. Mean +/- SD maternal age of mutant cases was 37.5 +/- 2.9, significantly greater than the value of 35.8 years in controls. A regression analysis indicated a rate of change of the log of the rate of about +0.20 with each year of maternal age between 30 and 45 years. When paternal age was introduced, the maternal age coefficient increased to about +0.25--close to that seen for 47, +21--but the paternal age coefficient was -0.06. After being matched for maternal age and year of diagnosis, the case-control difference in paternal age for 24 mutant cases was -2.4 with a 95% confidence interval of -4.6 to -0.1 years. In a regression analysis of the effects of both parental ages on the (log) rate, the maternal age coefficient was +0.25 and the paternal age coefficient was -0.06. These results are consistent with a (weak) negative paternal age effect in the face of a strong maternal age effect. Since ESAC include a heterogeneous group of abnormalities, the maternal age and paternal age trends, if not the result of statistical fluctuation or undetected biases, may involve different types of events. Data in the literature suggest that chromosomes with de novo duplicated inversions of 15p have a strong maternal age effect (but little paternal age effect). Such chromosomes, however, do not account for the active maternal age trends seen in the data analyzed here. Inherited ESAC exhibited no such trends.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benn P. A., Hsu L. Y. Incidence and significance of supernumerary marker chromosomes in prenatal diagnosis. Am J Hum Genet. 1984 Sep;36(5):1092–1102. [PMC free article] [PubMed] [Google Scholar]
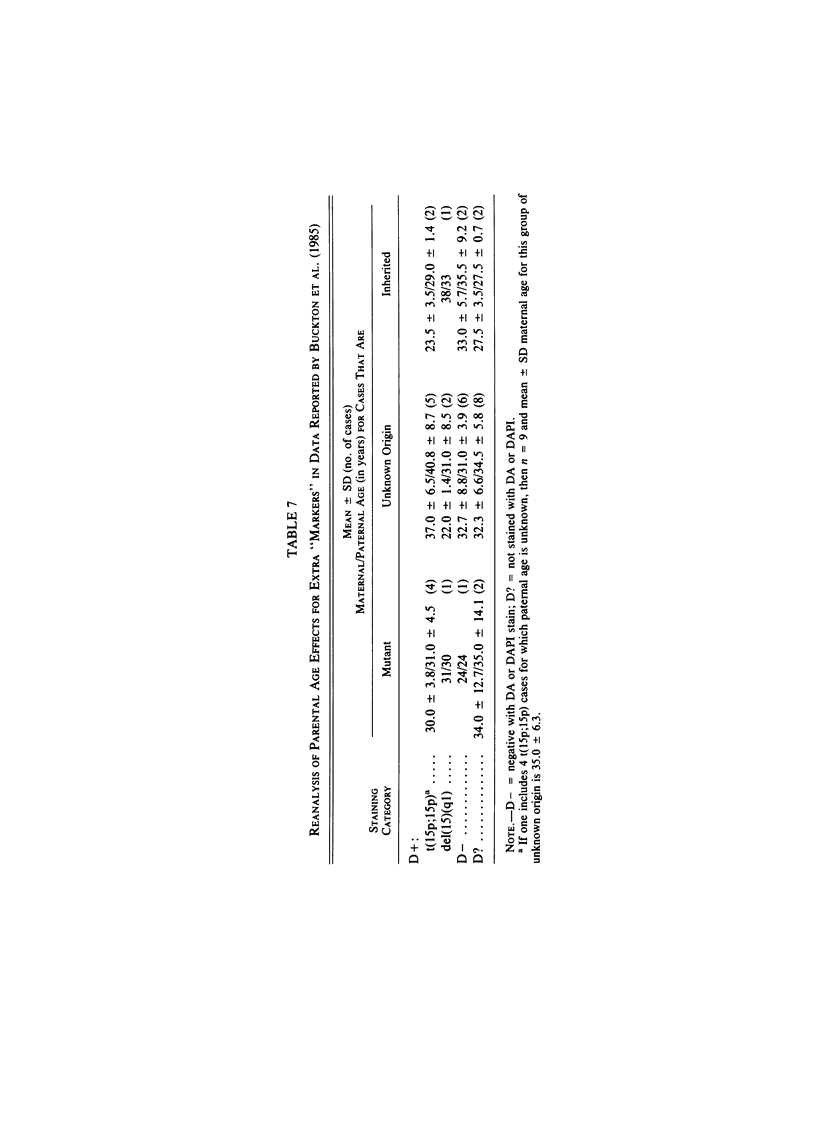
- Buckton K. E., Spowart G., Newton M. S., Evans H. J. Forty four probands with an additional "marker" chromosome. Hum Genet. 1985;69(4):353–370. doi: 10.1007/BF00291656. [DOI] [PubMed] [Google Scholar]
- Carothers A. D., Collyer S., De Mey R., Frackiewicz A. Parental age and birth order in the aetiology of some sex chromosome aneuploidies. Ann Hum Genet. 1978 Jan;41(3):277–287. doi: 10.1111/j.1469-1809.1978.tb01895.x. [DOI] [PubMed] [Google Scholar]
- Connor J. M., Gilmore D. H. An analysis of the parental age effect for inv dup (15). J Med Genet. 1984 Jun;21(3):213–214. doi: 10.1136/jmg.21.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook E. B., Cross P. K., Regal R. R. The frequency of 47,+21,47,+18, and 47,+13 at the uppermost extremes of maternal ages: results on 56,094 fetuses studied prenatally and comparisons with data on livebirths. Hum Genet. 1984;68(3):211–220. doi: 10.1007/BF00418391. [DOI] [PubMed] [Google Scholar]
- Hook E. B., Schreinemachers D. M., Willey A. M., Cross P. K. Rates of mutant structural chromosome rearrangements in human fetuses: data from prenatal cytogenetic studies and associations with maternal age and parental mutagen exposure. Am J Hum Genet. 1983 Jan;35(1):96–109. [PMC free article] [PubMed] [Google Scholar]
- Lieber E., Shah P., Hack M. Hazards of amniocentesis: an unidentifiable fragment. Clin Genet. 1978 Sep;14(3):130–132. doi: 10.1111/j.1399-0004.1978.tb02117.x. [DOI] [PubMed] [Google Scholar]
- Schreinemachers D. M., Cross P. K., Hook E. B. Rates of trisomies 21, 18, 13 and other chromosome abnormalities in about 20 000 prenatal studies compared with estimated rates in live births. Hum Genet. 1982;61(4):318–324. doi: 10.1007/BF00276595. [DOI] [PubMed] [Google Scholar]
- Sinha A. K., Linscombe V. A., Gollapudi B. B., Flake R. E., Park C. N. Supernumerary chromosomal elements in lymphocytes cultured from phenotypically normal human adult males. Cytobios. 1985;43(170):49–65. [PubMed] [Google Scholar]
- Toomey K. E., Mohandas T., Leisti J., Szalay G., Kaback M. M. Further delineation of the supernumerary chromosome in the Cat-Eye syndrome. Clin Genet. 1977 Nov;12(5):275–284. doi: 10.1111/j.1399-0004.1977.tb00941.x. [DOI] [PubMed] [Google Scholar]
- Wisniewski L., Hassold T., Heffelfinger J., Higgins J. V. Cytogenetic and clinical studies in five cases of inv dup(15). Hum Genet. 1979 Sep;50(3):259–270. doi: 10.1007/BF00399391. [DOI] [PubMed] [Google Scholar]


