Abstract
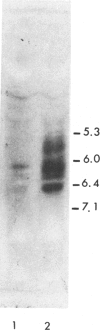
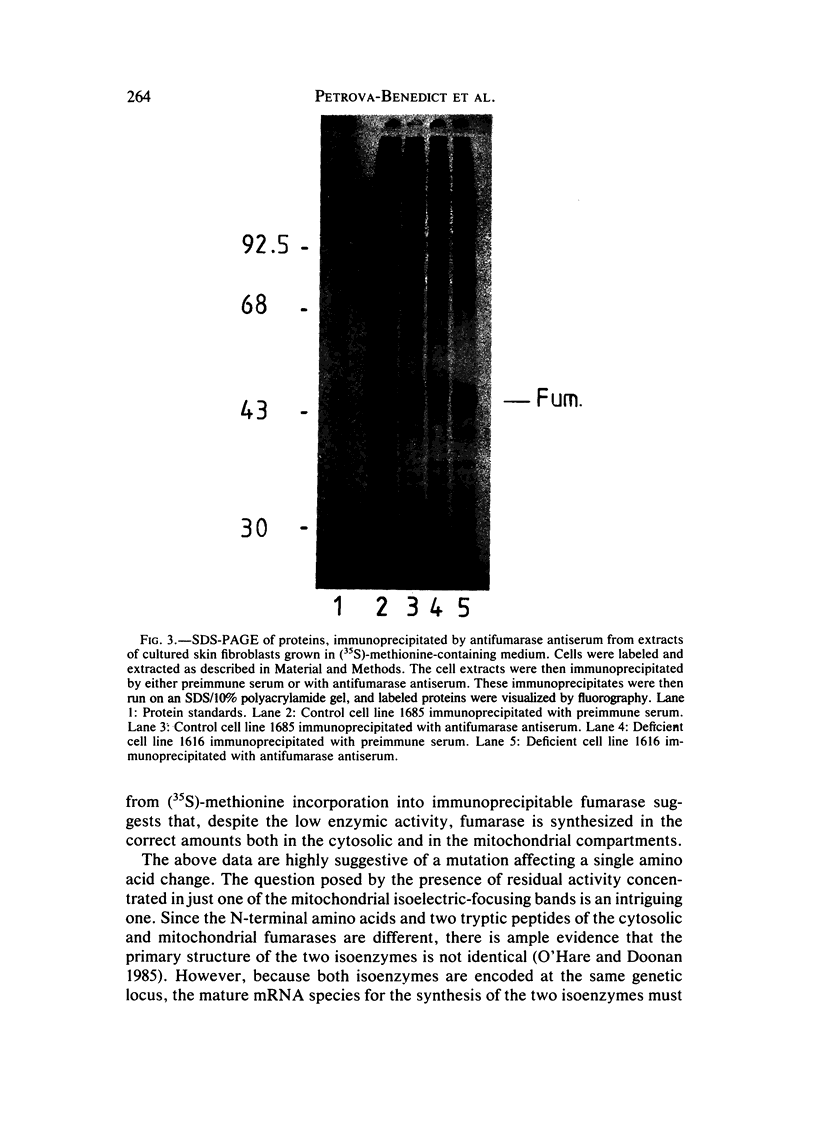
A male infant, whose parents were first cousins, presented at 6 mo of age with hypotonia, microcephaly, and delayed development. He was found to have large amounts of fumaric and succinic acids present in the urine. In lysed cultured skin-fibroblast preparations, the activity of fumarase was found to be 22.7% of that in controls. Cell fractionation by homogenization and by digitonin treatment indicated that the residual activity in the cells of the patient was primarily located in the mitochondrial fraction rather than in the cytosolic fraction. Isoelectric focusing of fibroblast extracts showed that six bands of fumarase activity were discernible in control cell lines, two of them cytosolic with pI's of 5.53 and 5.60 and four of them mitochondrial with a pI of 5.65-6.8. In contrast, isoelectric focusing of fibroblast extracts from the fumarase-deficient patient showed only a single band of activity with a pI corresponding to the mitochondrial type seen in the controls. Immunoprecipitation of proteins with rabbit antifumarase antibody in (35S)-methionine-labeled fibroblasts indicated that a protein of correct size (Mr = 44,000 daltons) corresponding to fumarase was synthesized in similar amounts in both the patients and controls. It is proposed that in the patient's cells a single active species of fumarase that is mitochondrial in location is synthesized. Since it is known that mitochondrial and cytosolic fumarases are encoded by the same gene but differ slightly in amino acid sequence, it is possible that a point mutation might explain these findings.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards Y. H., Hopkinson D. A. Further characterization of the human fumarase variant, FH 2--1. Ann Hum Genet. 1979 Oct;43(2):103–108. doi: 10.1111/j.1469-1809.1979.tb02002.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Yamanishi T., Tuboi S. Physicochemical, catalytic, and immunochemical properties of fumarases crystallized separately from mitochondrial and cytosolic fractions of rat liver. J Biochem. 1981 Jun;89(6):1923–1931. doi: 10.1093/oxfordjournals.jbchem.a133394. [DOI] [PubMed] [Google Scholar]
- Lam Hon Wah A. M., Lam K. F., Tsui F., Robinson B., Saunders M. E., Gravel R. A. Assignment of the alpha and beta chains of human propionyl-CoA carboxylase to genetic complementation groups. Am J Hum Genet. 1983 Sep;35(5):889–899. [PMC free article] [PubMed] [Google Scholar]
- O'Hare M. C., Doonan S. Purification and structural comparisons of the cytosolic and mitochondrial isoenzymes of fumarase from pig liver. Biochim Biophys Acta. 1985 Feb 4;827(2):127–134. doi: 10.1016/0167-4838(85)90080-9. [DOI] [PubMed] [Google Scholar]
- Ono H., Yoshimura N., Sato M., Tuboi S. Translocation of proteins into rat liver mitochondria. Existence of two different precursor polypeptides of liver fumarase and import of the precursor into mitochondria. J Biol Chem. 1985 Mar 25;260(6):3402–3407. [PubMed] [Google Scholar]
- Osborn M., Weber K. The detertent-resistant cytoskeleton of tissue culture cells includes the nucleus and the microfilament bundles. Exp Cell Res. 1977 May;106(2):339–349. doi: 10.1016/0014-4827(77)90179-3. [DOI] [PubMed] [Google Scholar]
- Tolley E., Craig I. Presence of two forms of fumarase (fumarate hydratase E.C. 4.2.1.2) in mammalian cells: immunological characterization and genetic analysis in somatic cell hybrids. Confirmation of the assignment of a gene necessary for the enzyme expression to human chromosome 1. Biochem Genet. 1975 Dec;13(11-12):867–883. doi: 10.1007/BF00484417. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Whelan D. T., Hill R. E., McClorry S. Fumaric aciduria: a new organic aciduria, associated with mental retardation and speech impairment. Clin Chim Acta. 1983 Aug 31;132(3):301–308. doi: 10.1016/0009-8981(83)90008-6. [DOI] [PubMed] [Google Scholar]