Abstract
Mice lacking the gene encoding the suppressor of cytokine signaling-1 (SOCS-1 −/−) and heterozygous for the IFN–γ gene (IFN-γ +/−) avoided the IFN-γ-dependent preweaning death of SOCS-1 −/− IFN-γ +/+ mice but did not exhibit the good health of young adult SOCS-1 −/− IFN-γ −/− mice. SOCS-1 −/− IFN-γ +/− mice died within 160 days of birth with massive T lymphocyte, macrophage, and eosinophil infiltration of all skeletal muscles and a similar severe myocarditis. The cornea also developed inflammatory infiltration and often a corneal ulcer. The mice exhibited evidence of selective CD8 T lymphocyte activation in populations in the thymus, spleen, and lymph nodes and focal T- and B-lymphoid infiltrates developed in the lung and salivary gland without apparent tissue damage. Comparison of SOCS-1 −/− IFN-γ +/− mice with various control mice indicated that the development of tissue-damaging T lymphocyte, macrophage, and eosinophil infiltrates required loss of SOCS-1 and the presence of some IFN–γ, but that the lung lymphoid infiltrates required only loss of SOCS-1 to develop.
The suppressor of cytokine signaling-1 (SOCS-1) is a cytokine-inducible cellular protein able to block or modulate signaling from a variety of cytokine receptors through inhibition of the JAK/STAT signaling pathway (1–4). Mice with homozygous inactivation of the SOCS-1 gene develop a neonatally fatal syndrome of fatty degeneration and necrosis of the liver, severe depletion of T and B lymphocytes, and tissue damage of the heart, lung, pancreas, and skin involving infiltration by macrophages and granulocytes, often of the eosinophil lineage (5–7). Development of this fatal neonatal syndrome can be prevented by administration of Abs to IFN-γ or by the generation of SOCS-1 −/− mice that also have deletion of the IFN-γ gene (SOCS-1 −/− IFN-γ −/− mice) (8). The latter animals at weaning exhibit normal hematopoietic tissues and no histological evidence of disease, identifying IFN-γ as being directly or indirectly mandatory for the development of this syndrome. Levels of IFN-γ in SOCS-1 −/− mice have variously been reported as normal or elevated (5, 7), with an additional suggestion that the disease might be initiated by excess IFN-γ production by aberrant activation of T lymphocytes (7).
In the course of generating SOCS-1 −/− IFN-γ −/− mice by crossmating, SOCS-1 −/− IFN-γ +/− mice were also produced, and these mice were observed to die as young adults, in contrast to the normal health exhibited by SOCS-1 −/− IFN-γ −/− mice of comparable age. The present studies were undertaken to establish whether the disease developing in SOCS-1 −/− IFN-γ +/− mice was merely a quantitative attenuation of the rapidly fatal disease in SOCS-1 −/− mice or whether the disease was qualitatively different in nature. Analysis has indicated that SOCS-1 −/− IFN-γ +/− mice develop a disease syndrome dominated by a profound myocarditis and polymyositis involving all skeletal muscles.
Materials and Methods
Mice.
The production of C57BL/6 × 129/Sv mice with homozygous inactivation of the SOCS-1 gene has been described elsewhere (5). Mice with homozygous inactivation of the IFN-γ gene were obtained from The Jackson Laboratory. SOCS-1 −/− IFN-γ +/− mice were generated initially by crossbreeding SOCS-1 +/− IFN-γ +/− mice and then usually by crossing SOCS-1 +/− IFN-γ +/+ with SOCS-1 −/− IFN-γ −/− mice. The genotype of progeny mice was established by Southern analysis of tail tips as described previously (5).
All mice were housed in clean animal rooms operated under barrier conditions.
Analysis.
Mice were anesthetized before performance of white cell and platelet counts, then killed by anesthesia. Spleen, peritoneal, and marrow populations were collected and analyzed as previously described (9). Organs were fixed in 10% formol/saline, sectioned, then stained routinely with hematoxylin and eosin.
Flow Cytometry.
Single cell suspensions of bone marrow, spleen, thymus, and lymph node were prepared, and red cells were lysed by incubation in 168 mM ammonium chloride. Cells were incubated with saturating amounts of 2.4G2 anti-Fcγ receptor Ab to reduce background staining, then variously with specific monoclonal Abs to the following murine cell surface antigens as described (10): CD4, CD8, Thy 1.2, CD44, MEL-14, IgM, Ly5-B220, Mac-1, F4/80, Gr-1, and Ter-119. The Abs were directly coupled to FITC or biotin, the latter being visualized with R-phycoerythrin-streptavidin. Analyses were performed on a FACScan cell sorter (Becton Dickinson) with dead cells and erythrocytes excluded by propidium iodide (1 μg/ml) staining and gating of forward angle and side scatter of light.
β-Galactosidase Staining.
Tissues were fixed for 4 h in 4% paraformaldehyde on ice, then washed three times each for 30 min in 100 mM sodium phosphate, pH 7.3, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, and stained overnight in wash buffer plus 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 1 mg/ml X-gal. Stained tissues were washed three times in PBS, postfixed overnight in 10% formalin, and then sectioned and counterstained with Nuclear Fast Red for microscopic examination.
Agar Cultures.
Agar cultures of marrow, spleen, and peritoneal cells were performed as described previously (9). The medium used was DMEM containing a final concentration of 20% supplemented newborn calf serum and 0.3% agar. Cultures contained 25,000 marrow cells or 50,000 spleen or peritoneal cells and were stimulated by 10 ng/ml of purified recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF), M-CSF, multipotential CSF (MultiCSF) (IL-3), 100 ng/ml murine stem cell factor (SCF), or 10 ng/ml purified recombinant human G-CSF. After 7 days of incubation at 37°C in a fully humidified atmosphere of 10% CO2 in air, quadruplicate replicate cultures were fixed by the addition of 1 ml 2.5% glutaraldehyde. The intact cultures were floated onto glass slides, dried, then stained in sequence for acetylcholinesterase, Luxol Fast Blue, and hematoxylin. Colony counts were then performed on the entire culture.
Immunohistochemistry.
Paraffin sections of 4% paraformaldehyde-fixed tissues were cut onto silane-coated slides. Immunolocalization using the Vectastain ABC kit (Vector Laboratories) was as per manufacturer's instructions. For CD3, the antigen was heat-retrieved in 1 mM EDTA (pH 8.0) The Abs used were rat primary Abs (anti-mouse CD45R, anti-human CD3, anti-mouse F4/80 or isotype controls; Serotec) and a biotinylated rabbit anti-rat secondary Ab (Vector Laboratories).
Results
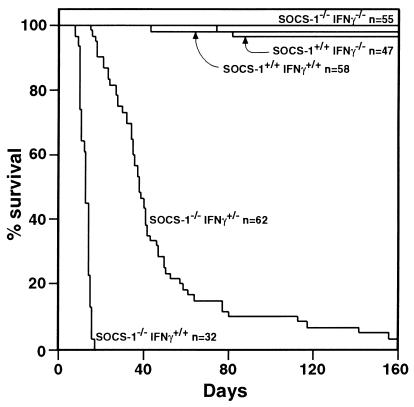
SOCS-1 −/− mice with a single functional IFN-γ allele (SOCS-1 −/− IFN-γ +/−) were born apparently healthy and in the expected Mendelian ratios but developed weight loss and weakness with a few dying just before weaning, and the majority dying between 30 and 70 days of age, with essentially all mice being dead by 160 days of age (Fig. 1).
Figure 1.
The delayed death of SOCS-1 −/− IFN-γ +/− mice compared with that of SOCS-1 −/− IFN-γ +/+ mice contrasts with the survival of SOCS-1 −/− IFN-γ −/−, SOCS-1 +/+ IFN-γ −/− and SOCS-1 +/+ IFN-γ +/+ mice. n = Number of mice.
Hematology.
Analysis was performed on 11–19 sick SOCS-1 −/− IFN-γ +/− mice aged from 20 to 140 days, comparing their hematological parameters with those of healthy SOCS-1 +/− IFN-γ +/− mice of the same age range. As shown in Table 1, SOCS-1 −/− IFN-γ +/− mice had a tendency for elevated neutrophil and monocyte levels but were neither anemic nor thrombocytopenic. Their spleen was variable in size and contained a slightly reduced percentage of lymphoid cells and an elevation of nucleated erythroid cells. Total marrow cell numbers were lower than in control mice, but this paralleled the smaller size of most of the sick SOCS-1 −/− IFN-γ +/− mice. In the marrow there was a slight reduction in the percentage of lymphocytes and a consistent increase in the percentage of eosinophilic cells at various stages of maturation. The thymus was of variable size but on average somewhat smaller than in control mice.
Table 1.
Hematological parameters in SOCS-1 −/− IFNγ +/− and SOCS-1 +/− IFNγ +/− mice
| SOCS-1 −/−IFNγ +/− Mice (n = 11–19) | SOCS-1 +/− IFNγ +/− Mice (n = 8) | |
|---|---|---|
| Blood | ||
| Total cells/μl | 8,590 ± 6,100 | 7,740 ± 2,300 |
| Neutrophils | 1,980 ± 1,080 | 950 ± 1,010 |
| Lymphocytes | 5,140 ± 4,690 | 6,270 ± 1,680 |
| Monocytes | 1,190 ± 850 | 330 ± 160 |
| Eosinophils | 290 ± 350 | 170 ± 110 |
| Hematocrit% | 45 ± 9 | 46 ± 2 |
| Platelets/μl | 932,240 ± 364,840 | 940,480 ± 102,960 |
| Marrow | ||
| Total cells per femur × 106 | 39.0 ± 8.9 | 50.3 ± 8.1 |
| Percent blasts | 4 ± 3 | 3 ± 1 |
| Myelocytes | 8 ± 3 | 9 ± 2 |
| Neutrophil/metamyelocytes | 34 ± 7 | 34 ± 8 |
| Lymphocytes | 14 ± 6 | 24 ± 9 |
| Monocytes | 8 ± 4 | 8 ± 2 |
| Eosinophils | 13 ± 8 | 5 ± 2 |
| Nucleated erythroid cells | 19 ± 9 | 17 ± 5 |
| Spleen (weight, mg) | 71 ± 55 | 89 ± 15 |
| Percent blasts | 4 ± 2 | 3 ± 1 |
| Myelocytes | 0.5 ± 0.8 | 0.4 ± 0.5 |
| Neutrophils | 5 ± 6 | 5 ± 6 |
| Lymphocytes | 67 ± 20 | 84 ± 5 |
| Monocytes | 4 ± 2 | 2 ± 1 |
| Eosinophils | 1 ± 2 | 1 ± 2 |
| Nucleated erythroid cells | 18 ± 19 | 4 ± 2 |
| Thymus (weight, mg) | 41 ± 26 | 76 ± 24 |
| Peritoneal Cells × 106 | 16.5 ± 9.4 | 13.4 ± 6.2 |
Figures are mean values ± SD.
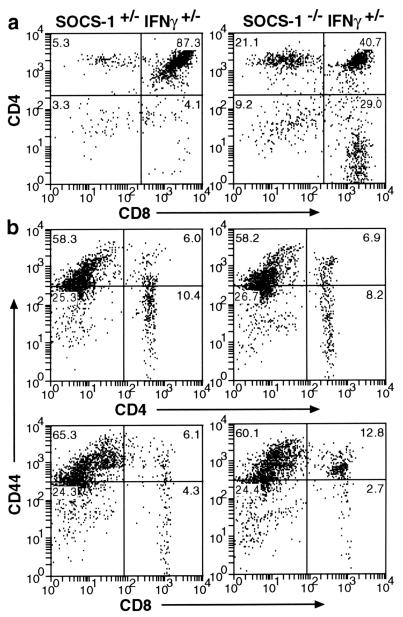
Flow cytometry analysis of tissues from SOCS-1 −/− IFN-γ +/− mice indicated an abnormally low percentage of CD4+ CD8+ cells and a corresponding rise in single positive cells in the thymus (Fig. 2). This deficiency increased as the animals became sicker and may merely have been the consequence of stress-induced involution. Using the activation marker CD44, a significantly increased proportion of activated Thy-1+ T lymphocytes was observed in the thymus, spleen, and the mesenteric node of each of five SOCS-1 −/− IFN-γ +/− mice compared with cells from SOCS-1 +/− IFN-γ +/− organs. Accompanying analysis with the MEL-14 marker, which is down-regulated on T cell activation, confirmed this observation (data not shown). Subset analysis in each of three mice revealed that activation was predominantly of the CD8+ T lymphocytes in each organ (Fig. 2). There was evidence of increased CD4+ T cell activation in some sick SOCS-1 −/− IFN-γ +/− mice, but to a significantly lesser degree than in the CD8+ compartment. Interestingly, the increased CD44 expression in CD8+ T lymphocytes was also evident in the spleen and mesenteric lymph node of healthy SOCS-1 −/− IFN-γ −/− mice, but not in wild-type or SOCS-1 +/+ IFN-γ −/− mice (data not shown). This suggests that increased activation may be an intrinsic property of T lymphocytes lacking SOCS-1 that is not dependent on disease development or the presence of IFN-γ. FACS analysis of the spleen and marrow confirmed the changes in the percentages of Ter-119-positive erythroid cells in the spleen and decreased B220-positive B lymphocyte percentages in the marrow as shown in Table 1.
Figure 2.
Flow cytometry analysis showing (a) the reduced percentage of CD4+ CD8+ lymphocytes in a SOCS-1 −/− IFN-γ +/− thymus compared with the normal profile in the thymus of a SOCS-1 +/− IFN-γ +/− mouse. (b) CD8+ but not CD4+ T-lymphocytes in the spleen of a SOCS-1 −/− IFN-γ +/− mouse show increased activation compared with cells in the control SOCS-1 +/− IFN-γ +/− spleen, as evidenced by increased expression of CD44.
In clonal cultures of marrow cells from sick SOCS-1 −/− IFN-γ +/− mice the overall numbers of colonies developing with various stimuli were comparable with those in control cultures of marrow cells from healthy SOCS-1 +/+ IFN-γ +/− and SOCS-1 −/− IFN-γ −/− mice (Table 2) as were the size, cellular composition, and maturation of the colonies. As with previously reported cultures of marrow cells from sick neonatal SOCS-1 −/− IFN-γ +/+ mice (9), cultures of adult SOCS-1 −/− IFN-γ +/− marrow cells also exhibited moderately elevated numbers of macrophage colonies and clusters, seen most clearly in cultures stimulated by M-CSF. In view of the consistently elevated percentages of eosinophils in the marrow of SOCS-1 −/− IFN-γ +/− mice and the massive numbers of eosinophils in the muscles of these mice (see below), the only oddity about the marrow cultures from these mice was the low frequency of eosinophil colonies developing either with GM-CSF or IL-3, with an actual absence of such colonies in cultures from some mice.
Table 2.
Clonal culture of marrow cells from SOCS-1 −/− IFNγ +/− mice
| Type | Stimulus | Blast | G | GM | M | Eo | Meg |
|---|---|---|---|---|---|---|---|
| SOCS-1 −/− | GM-CSF | 0.4 ± 0.5 | 22 ± 15 | 10 ± 3 | 64 ± 24 | 1 ± 2 | |
| IFNγ +/− | G-CSF | 18 ± 6 | 0 | 0.4 ± 0.7 | |||
| (n = 8) | M-CSF | 6 ± 2 | 9 ± 2 | 110 ± 12 | |||
| MultiCSF | 5 ± 2 | 28 ± 9 | 16 ± 7 | 45 ± 14 | 2 ± 2 | 3 ± 2 | |
| SCF | 8 ± 3 | 32 ± 6 | 1 ± 1 | 2 ± 1 | |||
| SOCS-1 +/+ | GM-CSF | 0 ± 0 | 21 ± 4 | 7 ± 4 | 47 ± 35 | 2 ± 2 | |
| IFNγ +/− | G-CSF | 11 ± 1 | 0 | 0 | |||
| (n = 2) | M-CSF | 4 ± 1 | 7 ± 0 | 82 ± 34 | |||
| MultiCSF | 5 ± 1 | 21 ± 0 | 15 ± 8 | 34 ± 27 | 3 ± 2 | 2 ± 1 | |
| SCF | 6 ± 2 | 24 ± 1 | 3 ± 1 | 0 | |||
| SOCS-1 −/− | GM-CSF | 1 ± 2 | 26 ± 10 | 8 ± 3 | 25 ± 13 | 8 ± 5 | |
| IFNγ −/− | G-CSF | 13 ± 5 | 0 | 0.3 ± 0.5 | |||
| (n = 6) | M-CSF | 4 ± 2 | 5 ± 1 | 71 ± 15 | |||
| MultiCSF | 4 ± 2 | 23 ± 8 | 17 ± 4 | 28 ± 10 | 5 ± 2 | 8 ± 5 | |
| SCF | 8 ± 5 | 24 ± 6 | 2 ± 1 | 1 ± 2 |
Figures are mean values of colony numbers per culture ± SD in 7 day cultures of 25,000 bone marrow cells. Blast, blast cell colony; G, granulocytic; GM, granulocyte–macrophage; M, macrophage; Eo, eosinophil; Meg, megakaryocytic colonies.
Pathology of SOCS-1 −/− IFN-γ +/− Mice.
Neonatal SOCS-1 −/− IFN-γ +/+ mice die with severe fatty degeneration and necrosis of liver cells, and inflammatory monocytic infiltrates in the liver, pancreas, skin, heart, and lung with characteristic epithelial thickening and keratosis of the skin. In contrast, neonatal SOCS-1 −/− IFN-γ −/− mice exhibit no tissue pathology (8).
As shown in Table 3 and Fig. 3, there were multiple lesions observable in sick SOCS-1 −/− IFN-γ +/− mice that formed a distinctive pattern in postweaning mice.
Table 3.
Frequency of pathological lesions in SOCS-1 −/− IFNγ +/− and control mice
| Parameter | Percentage of mice with various lesions
|
|||
|---|---|---|---|---|
| SOCS-1 −/−IFNγ +/−(n = 38) | SOCS-1 +/−IFNγ +/−(n = 11) | SOCS-1 −/−IFNγ −/−(n = 22) | SOCS-1 +/+ IFNγ −/−(n = 22) | |
| Muscle infiltration | ||||
| Massive | 79 | 0 | 0 | 0 |
| Minor | 18 | 0 | 0 | 0 |
| Heart infiltration | ||||
| Massive | 47 | 0 | 0 | 0 |
| Minor | 36 | 0 | 0 | 0 |
| Corneal infiltration | 68 | 0 | 0 | 0 |
| Ulcer | 39 | 0 | 0 | 0 |
| Marrow excess eosinophils | 68 | 10 | 50 | 0 |
| Lymphoid foci | ||||
| Lung | 97 | 0 | 64 | 0 |
| Salivary gland | 81 | 0 | 0 | 0 |
| Liver | 21 | 0 | 0 | 0 |
| Kidney | 24 | 0 | 0 | 0 |
| Excess plasma cells | 43 | 0 | 37 | 0 |
| Thymus cortex atrophy | 88 | 0 | 5 | 0 |
| Skin, patches of epithelial thickening | 43 | 9 | 0 | 0 |
| Liver, excess hematopoietic cells | 68 | 8 | 18 | 18 |
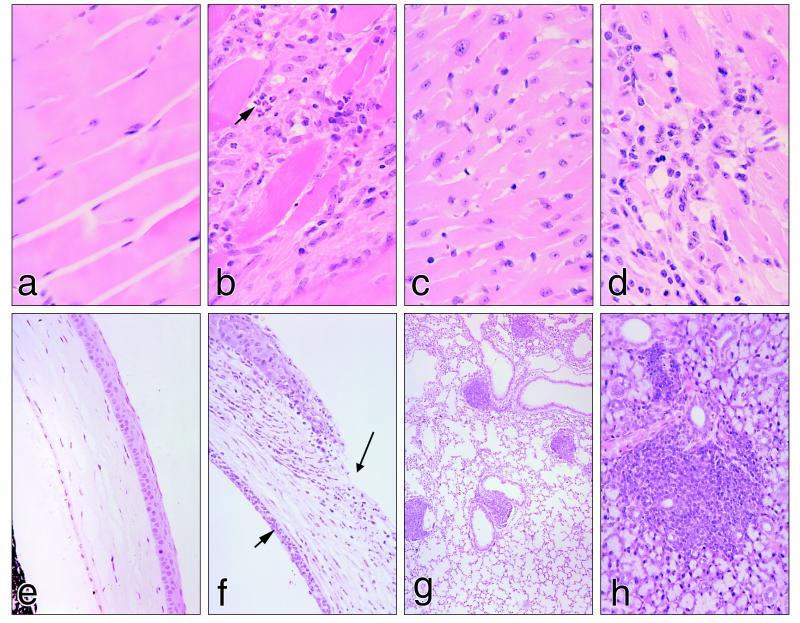
Figure 3.
Pathological lesions characteristic of SOCS-1 −/− IFN-γ +/− mice compared with normal tissues in control SOCS-1 +/− IFN-γ +/− mice: (a) normal skeletal muscle, (b) SOCS-1 −/− IFN-γ +/− skeletal muscle showing muscle cell destruction and extensive infiltration of T lymphocytes, macrophages, and eosinophils (arrow), (c) normal cardiac muscle, (d) cardiac tissue in a SOCS-1 −/− IFN-γ +/− mouse showing muscle cell destruction and infiltration by T lymphocytes, macrophages, and eosinophils, (e) normal cornea, (f) cornea of a SOCS-1 −/− IFN-γ +/− mouse showing an inflammatory infiltrate, a corneal ulcer (long arrow), and an accumulation of neutrophils in the anterior chamber (short arrow), (g) low power view of a SOCS-1 −/− IFN-γ +/− lung showing five focal lymphocyte infiltrates, all related to lung vessels, and (h) SOCS-1 −/− IFN-γ +/− salivary gland showing focal lymphocyte infiltration (H&E stain).
The most spectacular lesions involved skeletal muscle and the heart. Ninety-seven percent of SOCS-1 −/− IFN-γ +/− mice showed inflammatory infiltrates that involved all striated muscles. In most mice, these infiltrations were extremely extensive with virtually each muscle cell being individually separated by an infiltrating population mainly composed of CD3-positive T lymphocytes, F4/80-positive macrophages, and mature eosinophils. B lymphocytes were not involved in these infiltrates (Fig. 3). No mitotic activity was observed in these populations. Commonly, the muscle cells exhibited destruction of cytoplasm and nuclei with no evidence of regeneration.
In most animals, the heart also showed extensive inflammatory infiltration, most evident in the ventricles. This again involved T lymphocytes, macrophages, and mature eosinophils but sometimes also included neutrophils (Fig. 3). In some animals, the inflammatory infiltrate occupied as much as half the width of the ventricle wall and few surviving myocytes were visible in these regions (Fig. 3). Some mitotic activity was observable in infiltrated regions, in some cases appearing to involve cardiac myocytes. In a few instances, inflammatory infiltrates were confined to the subpericardial regions. The heart was not dilated as a consequence of these inflammatory changes.
In two-thirds of the animals, the cornea showed inflammatory infiltration by macrophages accompanied either by eosinophils or neutrophils. In sixty percent of such cornea, there was also loss of corneal epithelium and the presence of a corneal ulcer (Fig. 3). This was often accompanied by massive numbers of neutrophils in the aqueous humor adjacent to the cornea. The remainder of the eye usually appeared normal.
As shown in Table 3, the inflammatory infiltrates in muscle, heart, and cornea were restricted to mice of the SOCS-1 −/− IFN-γ +/− genotype. In these mice, there was also a quite distinct second type of tissue infiltrate in the form of focal lymphoid infiltrates, most prominently involving the lung and salivary glands. Typically, these lymphoid infiltrates were tight spherical aggregates which, in the lung, were commonly adjacent to vessels (Fig. 3). In neither organ was any damage apparent in the tissues adjacent to the lymphoid aggregates. In both tissues, the lymphoid foci contained CD45-positive B lymphocytes and more numerous CD3-positive T lymphocytes showing no mitotic activity. In 40% of the mice, there were increased numbers of plasma cells in the lymph nodes, often with some enlargement of the nodes. Less often in such mice, excess plasma cells were also evident in the red pulp of the spleen. The lymphoid foci in the lung and salivary glands of these latter mice sometimes contained small numbers of plasma cells. The thymus in SOCS-1 −/− IFN-γ +/− mice tended to be smaller than normal, and, although a cortex was usually present, it was thinner than in normal organs. The lymphoid follicles in the spleen of SOCS-1 −/− IFN-γ +/− mice usually contained no, or quite small, germinal centers. Occasionally, small lymphoid aggregates were also present in the liver, bladder wall, and kidney.
In the lungs of half of the mice there appeared to be excessive numbers of macrophages in the alveolar walls, and, occasionally, such cells were present as minor cuffs around the bronchi. The presence of these inflammatory macrophages appeared to be independent of the presence of lymphoid aggregates, the latter often being numerous and of large size in lungs that were otherwise quite normal (Fig. 3). Occasional SOCS-1 −/− IFN-γ +/− mice showed mixed macrophage and lymphoid infiltrates in the gut or bladder.
With the exception of one very young SOCS-1 −/− IFN-γ +/− mouse with minor fatty degeneration and necrosis of the liver, the parenchymal cells in the liver of SOCS-1 −/− IFN-γ +/− mice had a normal morphology and the only common abnormalities in these livers were a slight excess in the numbers of small mixed hematopoietic foci, which included T lymphocytes, usually related to hepatic vessels, and a slight excess in the number of Kupffer cells. In most SOCS-1 −/− IFN-γ +/− animals, the pancreas was normal in architecture. In the SOCS-1 −/− IFN-γ +/− mice, the skin was either normal or showed merely small foci of epithelial thickening and keratinization between areas of normal skin. T lymphocytes cells were present in excess numbers in the epithelium.
The marrow was characteristic in SOCS-1 −/− IFN-γ +/− mice in usually exhibiting prominent foci of immature and mature eosinophils.
As shown in Table 3, SOCS-1 +/− IFN-γ +/− mice in the same age range showed no histological abnormalities with the exception of one animal with some minor patches of skin epithelial thickening and keratinization. This indicated that the heterozygous state of IFN-γ, by itself, did not result in organ pathology. Similarly, in SOCS-1 +/+ IFN-γ −/− mice, no organ pathology was evident in mice of the age range surveyed, indicating that loss of the IFN-γ gene did not lead either to tissue inflammatory infiltration or to the development of lymphoid foci in the lung or salivary glands.
SOCS-1 −/− IFN-γ −/− mice exhibited good health in the age range surveyed (up to 6 mo). None showed inflammatory lesions in skeletal muscle or the heart. However, two-thirds had lymphoid foci in the lung, but, interestingly, none had lymphoid foci in the salivary glands, which were normal except for one animal that showed a large focus of eosinophils confined to the organ. The SOCS-1−/− IFN-γ −/− mice did, however, resemble SOCS −/− IFN-γ +/− mice in that half of the mice had abnormally large foci of eosinophils in the bone marrow, and one-third had excessive numbers of plasma cells.
Discussion
From the intermediate location of the mortality curve of SOCS-1 −/− IFN-γ +/− mice between those of SOCS-1 −/− IFN-γ +/+ and SOCS-1 −/− IFN-γ −/− mice, it can be concluded that mortality in SOCS-1 −/− mice is influenced by the level of IFN-γ present and presumably that IFN-γ levels in mice with a single functional IFN-γ allele must lie between those of IFN-γ −/− and +/+ mice. On this reasoning, it could have been anticipated that the pathology in SOCS-1 −/− IFN-γ +/− mice would be similar in general nature to that in SOCS-1 −/− IFN-γ +/+ mice, but in a more attenuated form.
The lethal neonatal syndrome in SOCS-1 −/− IFN-γ +/+ mice is dominated by massive fatty degeneration and necrosis of the liver with an associated lymphoid hypoplasia, macrophage infiltration of the pancreas, skin, lung, heart, and hyperplasia of skin epithelium (5). Massive liver destruction is known to occur in neonatal, but not adult, mice following the injection of large doses of IFN-γ (11, 12) and may reflect the peculiar susceptibility of the neonatal liver. If so, liver disease comparable with that in SOCS-1 −/− IFN-γ +/+ mice might not be evident in mice if lower IFN-γ levels delayed serious disease until after weaning. Indeed, in sharp distinction from SOCS-1 −/− IFN-γ +/+ mice, the SOCS-1 −/− IFN-γ +/− mice became moribund without major pathology in the liver or pancreas and with only minor macrophage infiltration in the lung and skin.
The diseases actually developing in SOCS-1 −/− IFN-γ +/− mice were highly distinctive and uniform in pattern, and puzzlingly appeared to involve two different types of pathological processes. Virtually all SOCS-1 −/− γIFN- +/− mice exhibited T lymphocyte, macrophage, and eosinophil infiltration of skeletal muscle and heart. Other organs involved by the same type of inflammatory infiltrate were the cornea and, much less frequently and less severely, the lung, bladder, and gut. These lesions contrasted sharply with the parallel development of lymphoid foci containing T and B lymphocytes in the lung and salivary gland associated often with excessive numbers of plasma cells in the lymph nodes and with occasional plasma cell foci in other organs. In most animals, there was some atrophy of the thymus cortex, sometimes with medullary enlargement.
The absence of T lymphocyte-macrophage-eosinophil inflammatory lesions in SOCS-1 −/− IFN-γ −/− and SOCS-1 +/+ IFN-γ −/− mice suggests that this pathology requires both the absence of SOCS-1 and the presence of at least some IFN-γ. In contrast, the lung T and B lymphoid infiltrates, which were also evident in SOCS-1 −/− IFN-γ −/− mice, appear to require the absence of SOCS-1 but not to be dependent on IFN-γ. T lymphocytes from SOCS-1 −/− mice are hyperresponsive to IL-2 (7) and, if activated in lung and salivary gland lymphoid foci, may produce other biologically active molecules to recruit B lymphocytes to these lesions. Alternatively, signaling from cytokines that regulate B cells might also be negatively regulated by SOCS-1.
The striking cellular infiltration and tissue damage in SOCS-1 −/− IFN-γ +/− mice was restricted to the muscles, heart, and cornea with only minor involvement of other tissues. This highly selective organ involvement suggests that these particular tissues may have been exhibiting serious dysfunction in the absence of SOCS-1 that encouraged attack by T lymphocytes, macrophages, and eosinophils. The targeted SOCS-1 allele includes a β-galactosidase gene that acts as a surrogate marker for SOCS-1 expression. An analysis in progress of β-galactosidase activity in the tissues of SOCS-1 −/− IFN-γ +/− mice has revealed that SOCS-1 is actively transcribed in both muscle and heart cells but not detectibly in the cornea. Loss of SOCS-1 might well therefore result in sufficient dysfunction at least in some tissues to attract attack by T lymphocytes, macrophages, and eosinophils.
T lymphocytes from SOCS-1 −/− mice have been reported to be abnormally responsive to mitotic stimulation (7) and marrow cells from SOCS-1 −/− mice induce death beginning 2 mo after transplantation to irradiated SOCS-1 +/+ mice (D.M. and W.A., unpublished data) or JAK3 −/− mice (7). Because disease development in SOCS-1 −/− mice is IFN-γ-dependent (8), these observations suggest that T lymphocytes could be initiating the inflammatory lesions in SOCS-1 −/− IFN-γ +/− mice by an autoaggressive process perhaps directed particularly against tissues failing to produce SOCS-1, with the involvement of macrophages and eosinophils being dependent on IFN-γ production by the activated T lymphocytes. However, macrophages from SOCS-1 −/− mice have also been shown to be abnormally reactive to IFN-γ (8, 9), and, therefore, the involvement of macrophages in the inflammatory lesions of SOCS-1 −/− IFN-γ +/− mice may not be wholly dependent on T lymphocyte dysfunction.
It is intriguing that activation of T lymphocytes in these mice appeared to be restricted largely to the CD8 subset, which suggests that the tissue antigens involved may be associated with Class I major histocompatibility complex molecules. Alternatively, if lack of SOCS-1 within T lymphocytes leads to dysfunction of the cells and autoaggression, this dysfunction may be restricted for some reason to CD8 cells—cells well recognized as exhibiting cytotoxic activity.
Future experiments may more clearly characterize the component processes operating to generate these various types of organ pathology. Meantime, the SOCS-1 −/− IFN-γ +/− mouse offers a spectacular model of spontaneous myocarditis, polymyositis and corneal ulcer development that should be of value for a variety of studies. It would also be of value to determine whether comparable diseases in humans might have a similar molecular basis.
Acknowledgments
We thank S. Mihajlovic, E. Tsui, N. Sprigg, J. Mighall, and K. Gray for skillful technical assistance throughout these studies.
Abbreviations
- SOCS-1
suppressor of cytokine signaling-1
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- G-CSF
granulocyte CSF
- M-CSF
macrophage CSF
- MultiCSF
multipotential colony-stimulating factor
- SCF
stem cell factor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160255197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160255197
References
- 1.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, et al. Nature (London) 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 2.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. Nature (London) 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 3.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al. Nature (London) 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson S E, Willson T A, Farley A, Starr R, Zhang J-G, Baca M, Alexander W S, Metcalf D, Hilton D J, Nicola N A. EMBO J. 1999;18:375–385. doi: 10.1093/emboj/18.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Starr R, Metcalf D, Elefanty A G, Brysha M, Willson T A, Nicola N A, Hilton D J, Alexander W A. Proc Natl Acad Sci USA. 1998;95:14395–14399. doi: 10.1073/pnas.95.24.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naka T, Matsumoto T, Narazaki M, Fujimoto A, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marine J-C, Topham D J, McKay C, Wang D, Parganas E, Stravopodis D, Yoshimura A, Ihle J N. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- 8.Alexander W S, Starr R, Fenner J E, Scott C L, Handman E, Sprigg N S, Corbin J E, Cornish A L, Darwiche R, Owczarek C M, et al. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf D, Alexander W S, Elefanty A G, Nicola N A, Hilton D J, Starr R, Mifsud S, Di Rago L. Leukemia. 1999;13:926–934. doi: 10.1038/sj.leu.2401440. [DOI] [PubMed] [Google Scholar]
- 10.Alexander W S, Roberts A R, Nicola N A, Li R, Metcalf D. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 11.Gresser I. Interferon. 1982;4:95–127. [PubMed] [Google Scholar]
- 12.Gresser I, Aguet M, Morel-Maroger L, Woodrow D, Puvion-Dutilleul F, Guillon J C, Maury C. Am J Pathol. 1981;102:396–402. [PMC free article] [PubMed] [Google Scholar]