Abstract
The sst2 somatostatin receptor mediates the antiproliferative effects of somatostatin analogs. The present study demonstrates that stable expression of sst2 in the hamster pancreatic cancer cells PC-1 and PC-1.0 activates an autocrine negative loop leading to an in vitro inhibition of cell proliferation. In vivo studies conducted in Syrian golden hamsters after orthotopic implantation of PC-1.0 cells showed that both tumor growth and metastatic progression of allografts containing 100% of sst2-expressing cells were significantly inhibited for up to 20 days after implantation, as compared with control allografts that did not express sst2. A local antitumor bystander effect was observed after induction of mixed tumors containing a 1:3 ratio of sst2-expressing cells to control cells. Tumor volume and incidence of metastases of mixed tumors were significantly reduced at day 13 post implantation. This effect decreased with time as at day 20, growth of mixed tumors was similar to that of control tumors. After administration of the cytotoxic somatostatin conjugate AN-238 on day 13, antitumor bystander effect observed in mixed tumors was significantly extended to day 20. We also observed that in vitro invasiveness of sst2-expressing PC-1.0 cells was significantly reduced. Tyrosine dephosphorylation of E-cadherin may participate in restoring the E-cadherin function, reducing in turn pancreatic cancer cell motility and invasiveness. This dephosphorylation depends on the tyrosine phosphatase src homology 2-containing tyrosine phosphatase 1 (SHP-1) positively coupled to sst2 receptor. The inhibitory effect of sst2 gene expression on pancreatic cancer growth and invasion combined with chemotherapy with targeted cytotoxic somatostatin analog administration provides a rationale for a therapeutic approach to gene therapy based on in vivo sst2 gene transfer.
Keywords: bystander effect, tumor invasion, targeted chemotherapeutic agent, experimental gene therapy, E-cadherin
Somatostatin is a widely distributed peptide that negatively regulates a number of cellular processes, including growth of multiple epithelial cell types (1). Somatostatin and its stable analog suppress the growth of various cancer cells, including neuroendocrine tumors that express somatostatin receptors (2, 3). The evidence indicates that direct antiproliferative effects of somatostatin and its analogs are mediated by specific cell surface receptors. Five subtypes of somatostatin receptors have been cloned from human, mouse, and rat (4). We found that, among them, the subtypes sst1, sst2, and sst5 mediate the antiproliferative effect of stable somatostatin analogues in vitro (5). We demonstrated that somatostatin subtype 2 (sst2) receptor mediated this antiproliferative effect through the association and stimulation of the tyrosine phosphatase src homology 2-containing tyrosine phosphatase 1 (SHP-1) activity (5, 6) and the subsequent arrest of cells in G0/G1 phase of cell cycle in response to an up-regulation of CDKI p27KIP1 expression and an increase in hypophosphorylated retinoblastoma protein level (7).
At present, pancreatic cancer is the fourth leading cause of cancer related deaths in Western countries. The only curative treatment of pancreatic cancer is a surgical resection. Unfortunately, a surgery for curative purposes is possible only in 10% to 15% of cases, and the overall five-year survival rate of pancreatic cancer is as low as 3.5%. To improve the effectiveness of treatment, an adjuvant therapy should be applied. In addition to new regimens of radio- or chemotherapy, gene therapy could be considered as a possible therapeutic tool for pancreatic cancer. We recently proposed an approach of gene therapy for pancreatic cancer based on the antioncogenic effects provided by the sst2 receptor gene expression (8). This approach was based on our previous demonstration that a specific loss of sst2 gene expression occurs in human pancreatic adenocarcinomas and in most of derived cell lines (9). These results correlate well with studies revealing that somatostatin-binding sites are either not detected or poorly detected in pancreatic adenocarcinomas by using both in vitro binding assay and in vivo scintigraphy or immunohistochemistry (1, 10). We postulated that the loss of sst2 expression in pancreatic cancer could confer a growth advantage in these tumors (9). This conclusion was subsequently strengthened by the correction of the sst2 defect in the human pancreatic cancer cell lines BxPC-3 and Capan-1 (11). Stable transfection of these cells with the human sst2 cDNA resulted in the induction of a negative-autocrine loop with secretion of endogenous ligand that activated constitutively the recombinant sst2 receptor. In vitro cell growth and both in vitro and in vivo tumorigenicity were significantly reduced in sst2-expressing cells (11). Experiments conducted in athymic mice demonstrated a dramatic decrease in tumor growth, as well as both local and distant antitumor bystander effects (11, 8). These results led us to conclude that (i) the human sst2 acts as a tumor suppressor in pancreatic cancer and (ii) the transfer of sst2 gene could represent a special therapeutic approach to this adenocarcinoma. Recently, we conducted preclinical studies demonstrating that somatostatin receptor-targeted chemotherapy caused inhibition of growth of primary tumors and their metastases. These results have been obtained after administration of the cytotoxic somatostatin analog AN-238 in mammary, brain, prostate, and renal cancer models expressing sst2 and sst5 receptors (12–16). In the present study, we used a transplantable model of pancreatic carcinoma in hamsters to investigate whether sst2 gene transfer in pancreatic cancer cells in combination with targeted cytotoxic somatostatin analog treatment could result in the inhibition of the primary tumor growth and metastatic progression. Molecular mechanisms involved in these effects were also investigated.
Materials and Methods
Peptides and Expression Vectors.
Somatostatin-14 and Tyr (11)-somatostatin-14 were from Novartis (Basel, Switzerland). Somatostatin analogs RC-160 and AN-238 (2-pyrrolinodoxorubicin linked to carrier RC-121) were synthesized as described (17, 18). Human sst2 cDNA was kindly provided by G. I. Bell (Howard Hughes Medical Institute, Chicago, IL.) and subcloned in pRS2 dicistronic mammalian expression vector (11). Mouse C453S-SHP-1 mutant (7) was subcloned in pcDNA3 vector and was a gift from C. Nahmias (ICGM Cochin, Paris).
Cell Culture and Transfections.
PC-1 cells were derived from pancreatic ductal carcinoma induced by N-nitrosobis(2-oxopropyl) amine in hamster (19). PC-1.0 cells derived from s.c. PC-1 tumors (20). The cells were cultured in RPMI medium 1640 containing 5% FCS, fungizone, streptomycin, and penicillin. PC-1 and PC-1.0 cells were transfected with pRS2 vector by using Lipofectamine reagent (GIBCO). Stable transfectants (named PC-1.0/sst2 and PC-1/sst2) were selected in 5% FCS RPMI medium 1640 containing 0.6 mg/ml Geneticin (G418; Sigma). Selected Geneticin-resistant clones were then cultured in medium containing 0.2 mg/ml and examined for their ability to bind [125I-Tyr11]somatostatin-14. Cells were concomitantly transfected with a mock vector (pSV2neo; Invitrogen) devoid of sst2 cDNA. These clones and wild-type cells were used as control cells. For transient transfections, PC1.0 and PC1.0/sst2 cells were grown in 100-mm diameter dishes (7.105 cells per dish) for 18 h in RPMI medium 1640 containing 5% FCS. After the medium was removed, cells were transfected in serum-free medium with 2 μg of pcDNA3/C453S-SHP-1 vector and 10 μl of polyethylenimine (Exgen 500 transfection reagent; Euromedex, Souffelweyersheim, France). After 3 h, FCS was added to the transfection medium (final concentration of 5%). Cells were then allowed to grow for 18 to 48 h before Western blotting or in vitro invasiveness assays, respectively. Cells transfected with pcDNA3 vector (Invitrogen) alone were used as control cells.
Binding Study.
Cell membranes were obtained from culture cells or from tumors as described (5, 21), and somatostatin binding was performed after acid washing by using labeled [125I-Tyr11]somatostatin-14 as described (5, 21). Binding assays were performed in triplicate in at least three separate experiments. Dissociation constant (Kd), maximal binding capacity (Bmax), and IC50 values were determined by using the nonlinear, least squares curve-fitting computer program, ligand, and GraphPad (San Diego) prism program.
Cell Growth Assay.
PC1.0 cells were plated in 35-mm diameter dishes at 4.104 cells/ml (2 ml per dish) in RPMI medium1640 containing 5% FCS. After a 12-h attachment phase, the medium was then replaced by fresh medium with or without FCS, and cells were cultured for 3 days. Cell growth was measured by cell counting by using Coulter counter model, Coulter Z1 (Coultronics France S.A.) (5).
Reverse Transcription (RT)-PCR Assays.
Total RNA was extracted by the guanidinium thiocyanate-phenol-chloroform method (9). RT-PCR was performed by using specific sense and antisense primers for rat preprosomatostatin, human sst2, and β actin (9, 11, 21).
Animals and Experimental Protocols.
Five-week-old male Syrian golden hamsters (weighing 70–80 g) were obtained from Harlan France (Gannat). They were kept in a temperature-controlled room with a 12-h light/12-h dark schedule and received pelleted diet and water. As described (22) with minor modifications, PC-1.0 cells were implanted orthotopically into hamsters. Briefly, under pentobarbital anesthesia and after a minilaparotomy, 5 × 105 PC-1.0 cells resuspended in 0.1 ml of FCS-free RPMI medium 1640 were injected into the tail of the pancreas under microscope (KAPS-SoM82, Bordeaux, France) by means of a sterile 29G lymphography catheter set (Vygon, Ecouen, France). Intrapancreatic allografts grew rapidly and were palpable 10–13 days after implantation. Because of the fast growth of tumors and their metastases, treatments were initiated within 20 days after inoculation. Three types of experiments were conducted by using groups of four to five animals.
Exp. 1.
PC-1.0, PC-1.0/neo and PC-1.0/sst2 cells were injected orthotopically into the pancreatic tail of Syrian golden hamsters to generate control and sst2 tumors. Tumor size was measured with calipers at 6, 9, 11, and 13 days post implantation after median laparotomy under pentobarbital anesthesia. Tumor volume was determined by the equation V = W2 × L/2, where W = width and L = length.
Exp. 2.
Growth curves of orthotopic allografts were obtained by inoculating mixed populations of PC-1.0/sst2 and PC-1.0 cells at a ratio of 25:75 (5 × 105 cells per allograft); these allografts were called mixed tumors. The tumor growth curves obtained were compared with those generated after injections of PC-1.0/sst2 cells (sst2 tumors) and PC-1.0 cells (control tumors). At day 13, tumor volume and metastases count were determined after median laparotomy.
Exp. 3.
Thirteen days after implantation, animals bearing control, sst2, or mixed tumors were divided into two groups each that received the following treatments as single i.v. injections: vehicle solution (5% glucose) or cytotoxic somatostatin conjugate AN-238 (100 nmol/kg). All of the injections were administered through the jugular vein under sodium pentobarbital anesthesia after tumor volume assessment and metastases count evaluations under median laparotomy. The effects of treatments were evaluated at day 20.
For each experiment, primary tumors or metastases were dissected and fixed in Dubosq Brazil medium for further histological analysis (8). Some tumors were also immediately placed in cold isopentane, frozen in liquid nitrogen, and then kept at −80°C for sst receptor binding or RT-PCR assays.
In Vitro Invasion Assay.
The invasive potential of cultured tumor cells was tested by using Matrigel-coated Transwell (Becton Dickinson) with 25-mm diameter polyvinylpyrrolidone-free polycarbonate filters of 8 μm size (Nalge; Nunc). Matrigel, diluted to 1:20 (250 μg/filter) with RPMI medium 1640, was used to coat filters (700 μl) and allowed to dry overnight under UV light. Cells (4 × 104) were plated on top of Matrigel in serum-free RPMI medium 1640 supplemented with 1% BSA. RPMI medium 1640 containing 10% FCS was placed on the bottom of the wells. Plates were incubated at 37°C for 48 h, and cells that invaded Matrigel and passed to the lower compartment of the filters were visualized by fixation with 3% (vol/vol) paraformaldehyde and staining with crystal violet at a final concentration of 0.2% (wt/vol). Then the stained cells were analyzed and counted under microscope (image-analysis system Visio-Lab 2000; Biocom, Paris).
Immunoprecipitation and Immunoblotting.
PC-1.0 and PC-1.0/sst2 cells transiently transfected or not with the pcDNA3/C453S-SHP-1 vector were cultured for 18 h. Then, cells were solubilized and immunoprecipitated or not with E-cadherin Abs (Transduction Laboratories, Montluçon, France) as described (6). Fifty micrograms of proteins were resolved through 7.5% SDS/polyacrylamide gels, transferred to a nitrocellulose membrane, and immunoblotted with anti-E-cadherin or anti-phosphotyrosine (Euromedex) or anti-SHP-1 Abs (Transduction Laboratories) as described (6). Immunoreactive proteins were visualized by the ECL immunodetection system (Pierce) and quantified by image analysis using Biocom apparatus.
Statistical Analysis.
Results are expressed as mean ± SEM. Statistical comparison was performed by using χ2, paired or unpaired Student t tests. P < 0.05 was considered significant.
Results
Constitutive Inhibition of PC-1.0 and PC-1 Pancreatic Cancer Cell Growth by sst2 Expression.
The human somatostatin receptor sst2 was stably expressed in PC-1.0 and PC-1 cells. One clone of PC-1.0/sst2 and two pools of PC-1/sst2 cells were selected. Using RT-PCR assay, we verified that PC-1.0/sst2 and PC-1/sst2 cells expressed human sst2 transgene at the mRNA level, whereas control cells did not (Fig. 1A, data not shown for PC-1 cells). Binding studies revealed that both wild-type control cells expressed endogenous somatostatin receptor. In PC-1.0 wild-type cells, a Bmax of 11 ± 1 fmol/mg and a Kd value of 0.8 ± 0.16 nM for [125I-Tyr11]somatostatin-14 were calculated. However, only low-affinity binding for somatostatin analog RC-160 was found (IC50, 0.6 ± 0.2 μM), indicating that sst2, sst3, and sst5 subtypes were not expressed endogenously. In displacement experiments, an IC50 value of 2.4 ± 0.9 nM could be determined with the selective sst1 analog CH-275, indicating that PC-1.0 cells possibly express the sst1 subtype. In PC-1.0/sst2 cells, somatostatin binding was increased to a Bmax value of 29 ± 3 fmol/mg. A high affinity for the somatostatin analog RC-160 was also observed, with an IC50 of 8 ± 1 nM indicating the presence of recombinant sst2 receptor at the protein level. Similar results were obtained with PC-1/sst2 pools (data not shown).
Figure 1.
Expression of human sst2 in hamster pancreatic cancer cells results in an autocrine negative loop. (A and B) PC1.0 cells stably expressing or not human sst2 receptor were cultured in 10-cm diameter dishes for 48 h in medium containing 5% FCS. Total RNA was extracted, and RT-PCR analysis was performed from clonal cell lines containing mock vector (1) or expressing human sst2 (2). The PCR products resulting from specific primers for human sst2 (A, 1107 pb), for preprosomatostatin (B, 487 pb), and β actin (A and B, 517 pb) were analyzed on polyacrylamide gels after ethidium bromide staining. M, DNA size marker (PGEM markers; Promega). RT-PCR carried out in the absence of reverse transcriptase during RT procedure were negative. Results are representative of two separate experiments. (C) Cells (4 × 104 per 35-mm diameter dish) were grown in medium supplemented with 5% FCS. After 16-h attachment phase, cells were cultured in serum-free medium for 3 days. Cell growth was measured at the indicated times by cell counting. Results are expressed as the cell number per dish (mean ± SE) and are representative of two separate experiments in triplicate (□, PC-1.0 cells; ■, PC-1.0/sst2 cells; Δ, PC-1 cells; and ▴, PC-1/sst2 cells).
Growth curves for sst2-expressing and control cells in vitro illustrate how the introduction of exogenous sst2 cDNA influences growth parameters. As shown in Fig. 1C, both PC-1.0 and PC-1 cells expressing sst2 and cultured in serum-free medium showed a significant reduction of cell growth in the absence of exogenous ligand, as compared with their respective control cells. After 2 days of culture, PC-1.0/sst2 and PC-1/sst2 cell proliferation was reduced by 32% ± 6% and 42% ± 15% (from three separates experiments in triplicate, P < 0.01). Similar results were obtained in serum-containing medium (data not shown). To verify whether the constitutive inhibition of PC-1.0 and PC-1 cell growth by sst2 expression was induced by production of somatostatin, we examined the expression of preprosomatostatin mRNA by RT-PCR analysis. When cells were cultured for 48 h, the level of preprosomatostatin transcripts was up-regulated in sst2-expressing PC-1.0 cells as compared with control cells (Fig. 1B). Similar results were observed for PC-1/sst2 cells (data not shown from analysis performed at 24, 48, and 72 h of culture).
The Expression of sst2 Receptor Inhibits Growth of Pancreatic Cancer in Vivo.
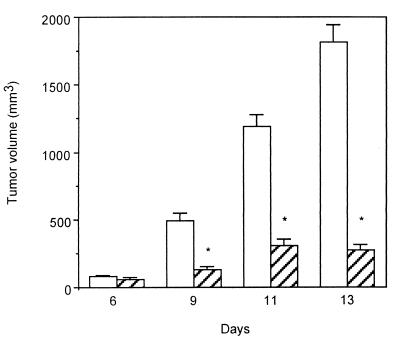
As shown in Fig. 2, PC-1.0 control cells allografted orthotopically into the tail of pancreas grew rapidly and exponentially. Similar results were obtained after implantation of PC-1.0/neo control cells (data not shown). In contrast, growth of sst2 tumors (generated after inoculation of PC-1.0/sst2 cells) was dramatically inhibited and remained under 300 mm3 in size until 13 days after inoculation.
Figure 2.
Effect of human sst2 expression on pancreatic cancer growth in hamsters. A total of 5 × 105 PC-1.0 (open bars) or PC-1.0/sst2 (hatched bars) cells resuspended in 0.1 ml FCS-free RPMI medium 1640 were injected into the tail of the pancreas of Syrian golden hamsters (Exp. 1). The tumor's size was measured after laparatomy at indicated days post cell implantation. Results are means ± SE from four animals per group and per day of examination and are representative of two separate experiments (*, P < 0.001).
The Expression of sst2 Receptor Induces Local Antitumor Bystander Effect in Pancreatic Primary Mixed Tumors.
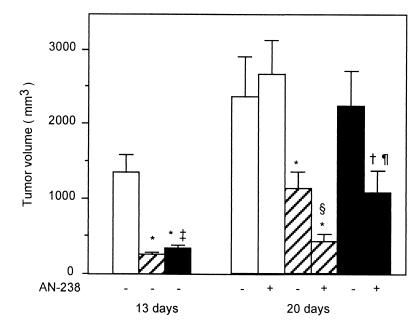
To assess whether the antioncogenic effect evoked by sst2 expression in PC-1.0 cells can influence the neighboring cells, by a so-called “bystander effect,” mixed allografts containing 25%:75% ratio of PC-1.0/sst2 cells to PC-1.0 cells were inoculated into the tail of pancreas. As shown in Fig. 3 Left, growth of primary pancreatic mixed tumors was significantly inhibited as compared with control tumors. However, the volume of mixed tumors remained significantly higher than that of exclusively sst2 tumors.
Figure 3.
Effects of a single i.v. injection of cytotoxic somatostatin conjugate AN-238 on primary pancreatic cancer growth in hamsters. PC-1.0 (open bars), PC-1.0/sst2 (hatched bars), and a mixture of PC-1.0/sst2 and PC-1.0 cells (ratio of PC-1.0/sst2 to PC-1.0 = 25:75; filled bars) were inoculated orthotopically into the tail of the pancreas of hamster (5 × 105 per site). Tumor volumes were measured at day 13 as described for Exp. 2 and at day 20 in group that did (+) or did not (−) receive single i.v. injections of 100 nmol/kg of AN-238 at day 13 according to Exp. 3. Values are from 20 animals at day 13 from four separate experiments and from 10 animals per group at day 20 from two separate experiments. Difference from control tumors (open bar): *, P < 0.001; †, P < 0.01. Difference between sst2 and mixed tumor volume at 13 days: ‡, P < 0.05. Difference with tumor volume at 20 days in untreated animals: §, P < 0.01; ¶, P < 0.05.
Sensitization to Chemotherapy with a Targeted Cytotoxic Somatostatin Conjugate AN-238 by sst2 Gene Transfer.
As shown in Fig. 3 Right, 20 days after implantation, the volume of pancreatic mixed tumors was not different from that of control tumors. The volume of sst2 tumors was significantly reduced when compared with control tumors but was significantly higher than that of sst2 tumors at 13 days after implantation (P = 0.0079, paired t test). RT-PCR confirmed the presence of mRNA for sst2 in tumor samples (sst2 tumors) at day 13 (data not shown). Binding study was also performed on membranes isolated from control and mixed tumor samples at day 13 after inoculation. No specific binding could be found on membranes from control tumors, whereas membranes from mixed tumors expressed specific binding for the analog RC-160 (data not shown). Thus, hamsters bearing control, sst2, or mixed tumors were injected 13 days after intrapancreatic tumor cell inoculation with AN-238 (2-pyrrolinodoxorubicin linked to carrier RC-121) or vehicle. As shown in Fig. 3, after administration of AN-238, volumes of both sst2 and mixed tumors at day 20 were significantly lower than that of the control tumors. However, despite the administration of AN-238, the volumes of mixed and sst2 tumors continued to increase significantly as compared with their initial volume on day 13 (0.0178 < P < 0.0033, paired-t test).
The Expression of sst2 Receptor Is Responsible for the Inhibition of Metastatic Progression of Pancreatic Carcinoma Established in Hamster.
A summary of the incidence of metastases at days 13 and 20 is given in Table 1. On day 13, the incidence of both distal and proximal histologically proven metastases was significantly reduced in hamsters bearing sst2 and mixed tumors, as compared with animals bearing control tumors. On day 20, in untreated animals, this decrease remained significant only for sst2 tumors. In animals treated with AN-238, incidence of metastases was lower for sst2 tumors, as compared with untreated animals. However, AN-238 administration did not reduce the rate of metastases accompanying control or mixed tumors on day 20.
Table 1.
Incidence of metastases after intrapancreatic implantation of PC-1.0 (control tumors), PC-1.0/sst2 (sst2 tumors), and a mixed population of PC-1.0/sst2∶PC-1.0 cells (25%∶75%, mixed tumors)
| Groups | Metastases on day 13 | Metastases on day 20
|
|
|---|---|---|---|
| − AN-238 | + AN-238 | ||
| Control | P, 95%; D, 81% (n = 22) | P, 100%; D, 100% (n = 10) | P, 90%; D, 100% (n = 10) |
| sst2 | P, 19%*; D, 19% (n = 26)* | P, 70%*; D, 50% (n = 14)† | P, 36%*‡; D, 36% (n = 14)* |
| Mixed | P, 43%*; D, 37% (n = 30)* | P, 90%; D, 100% (n = 10) | P, 100%; D, 80% (n = 10) |
Results are expressed as percentage of proximal (regional lymph nodes, spleen, stomach, and epiploon) and distal (liver and peritoneum) metastases at 13 and 20 days after implantation, with (+ AN-238) or without (− AN-238) a single i.v. administration of AN-238 analog on day 13. Cumulative results obtained from experiments 1, 2 (day 13; Figs. 2 and 3), and 3 (days 13 and 20; Fig. 3) are given (number of animals evaluated is indicated in parenthesis). P, proximal metastases; D, distal metastases. Difference with control: *, P < 0.001;
, P < 0.05. Difference with untreated animals:
, P < 0.05 (χ2 test).
The Expression of sst2 Receptor Is Responsible for the Inhibition of in Vitro PC-1.0 Cell Invasiveness.
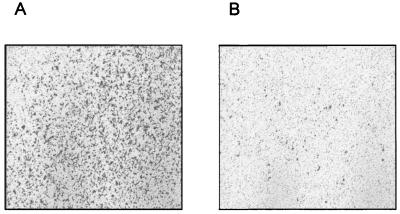
To characterize the inhibitory effect of sst2 expression on the invasiveness of pancreatic cancer cells, the ability to PC-1.0 cells to migrate through a Matrigel-coated filter was assessed. As shown in Fig. 4, a dramatic decrease of PC-1.0/sst2 cell invasiveness was observed, as compared with PC-1.0 cells in the absence of exogenous somatostatin ligand. Quantitative analysis from four separate experiments revealed that the invasion of sst2-expressing cells was reduced by 55% ± 5% and 49% ± 8%, when compared with PC-1.0 and PC-1.0/neo cells, respectively.
Figure 4.
Effect of sst2 expression on in vitro invasivenes of pancreatic cancer cells. Cells were plated in chamber containing a Matrigel-coated filter at its base. The chambers were immersed in RPMI medium 1640 with 1% of BSA for 30 min at 37°C. After 48-h incubation, the noninvasive cells, which remain on the topside of the filter, were removed, and the invasive cells, which attach themselves to the underside of the filter, were stained with 0.2% crystal violet after fixing with 3% paraformaldehyde. The figure represents a photograph at a low magnification (×40) of filters with stained cells (A, PC-1.0 cells; B, PC-1.0/sst2 cells) and is representative of four separate experiments.
E-Cadherin Is Dephosphorylated on Tyrosine Residues in Pancreatic Cancer Cells Expressing sst2 Receptor by a Mechanism Involving the Tyrosine Phosphatase SHP-1.
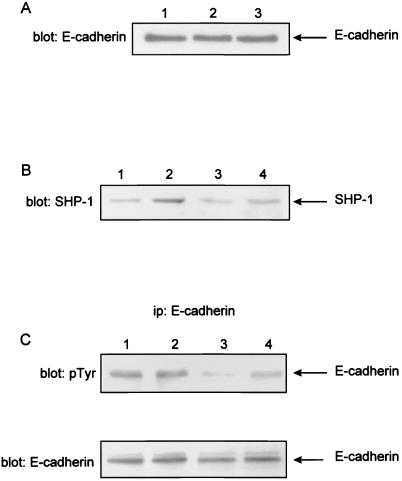
An altered pattern of E-cadherin expression and/or function has been associated with cell invasiveness and poor differentiation and/or prognosis of pancreatic cancer. As shown in Fig. 5A, no difference could be detected between E-cadherin expression of PC-1.0/sst2 cells and PC-1.0 or PC-1.0/neo control cells. Results were identical, the cells being cultured for 24 or 48 h (data not shown). However, as shown in Fig. 5C Upper, phosphorylation of E-cadherin on tyrosine residues was reduced by 45% ± 5% in sst2-expressing cells when compared with PC-1.0 or PC-1.0/neo control cells (five separate experiments). Fig. 5B shows that transfection of C453S-SHP-1 mutant is accompanied by an increase of SHP-1 expression. Transient expression of dominant negative SHP-1 mutant form in PC-1.0/sst2 cells reversed the hypophosphorylation of E-cadherin on tyrosine residues by 50% ± 4% (Fig. 5C Upper). No effect of SHP-1 mutant has been observed on PC-1.0. The blots were reprobed with anti-E-cadherin Abs to ensure that comparable amounts of E-cadherin were immunoprecipitatd at each points (Fig. 5C Lower). We also observed that transient transfection of the SHP-1 mutant in PC-1.0/sst2 cells restores the invasiveness of PC-1.0 control cells (data not shown).
Figure 5.
Effect of sst2 expression on E-cadherin protein expression and tyrosine dephosphorylation in pancreatic cancer cells. (A) Cells were cultured in 10-cm diameter dishes in medium containing 5% FCS. After a 12-h attachment phase, cells were cultured in serum-free medium for 24 h. Soluble proteins were subjected to SDS/polyacrylamide gel electrophoresis and immunoblotted (blot E-cadherin) with anti-E-cadherin Abs. Lane 1, PC1.0/neo cells; lane 2, PC-1.0 cells; and lane 3, PC1.0/sst2 cells. The arrow indicates the position of E-cadherin (120 kDa). Results are representative of three independent experiments. (B and C) PC-1.0 and PC-1.0/sst2 cells transiently transfected or not with the pcDNA3/C453S-SHP-1 vector (dominant negative SHP-1 mutant) were cultured in 100-mm dishes (7 × 105) cells/dish in RPMI medium 1640 supplemented with 5% FCS for 18 h. Cells were thereafter solubilized and immunoprecipitated or not with E-cadherin Abs. Solubilized proteins were resolved through 7.5% SDS/polyacrylamide gels and immunoblotted with anti-SHP-1 Abs (B, blot SHP-1). Immunoprecipitated proteins (ip: E-cadherin) were immunoblotted with anti-phosphotyrosine (C, blot: p-Tyr) or anti-E-cadherin (C, blot: E-cadherin, reblotting experiment). The arrow indicates the position of E-cadherin phosphorylation (120 kDa). Lanes 1, PC-1.0 cells; lanes 2, PC-1.0-SHP-1/C453S cells; lanes 3, PC-1.0/sst2 cells; lanes 4, PC-1.0/sst2-SHP-1/C453S cells. The figure is representative of three independent experiments.
Discussion
In the present study, we demonstrated that the expression of sst2 gene in hamster pancreatic cancer cells activated an autocrine negative loop that was responsible for an inhibition of cell growth in vitro. This effect was accompanied by an antioncogenic effect observed in vivo after orthotopic allotransplantation of recombinant cells. In a parallel manner, an antitumor bystander effect was observed in primary tumors containing a mixed population of cancer cells, among which only 25% expressed sst2 receptor, the growth of mixed tumors being significantly reduced as compared with controls that did not express sst2. The mechanism of this bystander effect is unknown, but, in the nude mouse model, we postulated that a local bystander effect could result from sst2 expression that in turn would increase local production of somatostatin and induce apoptosis by a negative autocrine loop (8). In the present study, we did not find a significant increase of serum somatostatin immunoreactivity in any of the tumor-bearing hamsters at different times of observation (data not shown). However, paracrine effect of somatostatin produced by sst2-expressing cells could play a major role in our model. Locally produced somatostatin could inhibit the release of growth factors and bind to sst1 receptor that is possibly expressed endogenously in PC-1.0 cells and that mediates the antiproliferative effect of somatostatin-14 (5, 8). Moreover, we found somatostatin binding sites for somatostatin analogue RC-160 in mixed tumors. This binding could account for sst2 transgene expression, but other subtypes such as sst5 may also be involved, the expression of which can be increased after chronic exposure of cells to somatostatin ligand (8). It is well known that, in gene therapy approach for solid cancers, the transfer of the therapeutic gene in all tumor cells is unlikely. A bystander killing effect on nontransduced cells as well as a long-term antitumor effect are thus required for the success of the cancer gene therapy. In our model, a significant antitumor bystander effect was reached when only 25% of tumor cells expressed sst2. However, this effect decreased in time. Because on day 13 after implantation both sst2 and mixed tumors still expressed somatostatin receptors, we administered the cytotoxic somatostatin analog AN-238 at dose of 100 nmol/kg. As result, both antioncogenic and antitumor bystander effects could be observed for sst2 and mixed primary pancreatic tumors even 20 days after implantation. However, control tumors, which did not express somatostatin receptors at day 13, showed no response to AN-238 treatment. The mechanism of action of AN-238 in hamster pancreatic cancer cells remains unknown. It probably binds to tumor membrane receptors such as sst2 or sst5 that are not occupied by somatostatin ligand produced by the autocrine loop. This binding results in an accumulation of its cytotoxic radical, 2-pyrrolinodoxorubicine (12–16, 23). The significant antitumor effect on primary pancreatic tumors in our model may be important for designing a therapeutic strategy by using sensitization to chemotherapy by sst2 gene transfer.
In addition to the antitumor effect on primary tumors, sst2 expression led also to an inhibition of metastatic progression observed on day 13 both for sst2 and mixed tumors. AN-238 analog administration extended this effect for sst2 tumors, but not for mixed tumors. These latter results suggest that metastases already present on day 13 or newly formed were not sensitive to cytotoxic somatostatin conjugate. Moreover, the critical mass of cells that become invasive could be higher in mixed tumors than in sst2 tumors after 13 days of growth.
In a view of the high invasive potential of pancreatic carcinoma, the inhibitory effect of sst2 expression on pancreatic cancer cell invasion is most important in planning a strategy for gene therapy. We tried to study the mechanism involved in this effect. In epithelial cells, intercellular adhesion is mediated by E-cadherin, a 120-kDa transmembrane glycoprotein localized at the adherens junctions. E-cadherin interacts with the actin cytoskeleton through cytoplasmic proteins named α, β, and γ catenins. The dysfunction and/or the loss of Ecadherin/catenins complex has been strongly implicated in the carcinogenesis and invasiveness of cancerous epithelial cells, including pancreatic cancer cells (24–26). The complex can be negatively regulated at different levels with mutation, loss of expression, and tyrosine phosphorylation. However, sst2 expression did not affect E-cadherin protein level in PC-1.0 cells. Regulation of E-cadherin function is not well understood, but a decrease in tyrosine phosphorylation seems to stabilize cadherin-dependent cell contacts (27, 28). We observed here that phosphorylation of E-cadherin on tyrosine residues was reduced in sst2-expressing cells. Regarding the reversing effect of dominant negative SHP-1 mutant expression, dephosphorylation of E-cadherin seemed to depend on the protein tyrosine phosphatase SHP-1 known to be positively coupled to sst2 receptor (6). Moreover, the fact that transient transfection of the SHP-1 mutant in sst2-expressing cells restores the cell invasiveness, suggests that tyrosine dephosphorylation is also implicated in the inhibition of invasiveness induced by sst2 expression. We can postulate that activation of tyrosine phosphatase SHP-1 coupled to sst2 may dephosphorylate and thus restore E-cadherin function that reduces pancreatic cancer cell motility and invasiveness.
In conclusion, the inhibitory effect on pancreatic cancer growth and invasiveness induced by sst2 receptor gene expression provide a rationale for developing a therapeutic approach to gene therapy. In addition, sst2 gene transfer in pancreatic carcinomas may result in sensitization with a targeted cytotoxic somatostatin analog improving the outcome of systemic treatment of this malignancy.
Acknowledgments
This paper was supported in part by grants from Ligue Nationale Contre le Cancer (Grant 257 8DB06D), Conseil Régional Midi Pyrénées (Grant 2AC FH013C), Association pour la Recherche contre le Cancer (Grant 5576), Groupe de Recherche de l'Institut Claudius Regaud 1999, and European Community Contract QLG3-CT-1999-0908.
Abbreviations
- sst
cloned somatostatin receptor subtype
- SHP-1
src homology 2-containing tyrosine phosphatase 1
- RT-PCR
reverse transcription–PCR
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.130196697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.130196697
References
- 1.Lamberts S W, Krenning E P, Reubi J C. Endocr Rev. 1991;12:450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- 2.Pollak M N, Schally A V. Proc Soc Exp Biol Med. 1998;217:143–152. doi: 10.3181/00379727-217-44216. [DOI] [PubMed] [Google Scholar]
- 3.Arnold R, Frank M. Digestion. 1996;57, Suppl. 1:69–71. doi: 10.1159/000201400. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer D, Bell G I, Berelowitz M, Epelbaum J, Feniuk W, Humphrey P P, AM, O C, Patel Y C, Schonbrunn A, Taylor J E, Reisine T. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 5.Buscail L, Esteve J P, Saint-Laurent N, Bertrand V, Reisine T, AM, O C, Bell G I, Schally A V, Vaysse N, Susini C. Proc Natl Acad Sci USA. 1995;92:1580–1584. doi: 10.1073/pnas.92.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez F, Esteve J P, Buscail L, Delesque N, Saint-Laurent N, Theveniau M, Nahmias C, Vaysse N, Susini C. J Biol Chem. 1997;272:24448–24454. doi: 10.1074/jbc.272.39.24448. [DOI] [PubMed] [Google Scholar]
- 7.Pages P, Benali N, Saint-Laurent N, Esteve J P, Schally A V, Tkaczuk J, Vaysse N, Susini C, Buscail L. J Biol Chem. 1999;274:15186–15193. doi: 10.1074/jbc.274.21.15186. [DOI] [PubMed] [Google Scholar]
- 8.Rochaix P, Delesque N, Esteve J P, Saint-Laurent N, Voight J J, Vaysse N, Susini C, Buscail L. Hum Gene Ther. 1999;10:995–1008. doi: 10.1089/10430349950018391. [DOI] [PubMed] [Google Scholar]
- 9.Buscail L, Saint-Laurent N, Chastre E, Vaillant J C, Gespach C, Capella G, Kalthoff H, Lluis F, Vaysse N, Susini C. Cancer Res. 1996;56:1823–1827. [PubMed] [Google Scholar]
- 10.Reubi J C, Kappeler A, Waser B, Laissue J A, Hipkin R W, Schonbrunn A. Am J Pathol. 1999;153:233–245. doi: 10.1016/S0002-9440(10)65564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delesque N, Buscail L, Esteve J P, Saint-Laurent N, Muller C, Weckbecker G, Bruns C, Vaysse N, Susini C. Cancer Res. 1997;57:956–962. [PubMed] [Google Scholar]
- 12.Koppan M, Nagy A, Schally A V, Arencibia J M, Plonowski A, Halmos G. Cancer Res. 1998;58:4132–4137. [PubMed] [Google Scholar]
- 13.Plonowski A, Schally A V, Nagy A, Sun B, Szepeshazi K. Cancer Res. 1999;59:1947–1953. [PubMed] [Google Scholar]
- 14.Kahán Z, Nagy A, Schally A V, Hebert F, Sun B, Groot K, Halmos G. Int J Cancer. 1999;82:592–598. doi: 10.1002/(sici)1097-0215(19990812)82:4<592::aid-ijc20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Kiaris H, Schally A V, Nagy A, Sun B, Szepeshazi K, Halmos G. Clin Cancer Res. 2000;6:709–717. [PubMed] [Google Scholar]
- 16.Plonowski, A., Schally, A. V., Nagy, A., Kiaris, H., Hebert, F. & Halmos, G. (2000) Cancer Res., in press. [PubMed]
- 17.Cai R Z, Szoke B, Lu R, Fu D, Redding T W, Schally A V. Proc Natl Acad Sci USA. 1986;83:1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagy A, Schally A V, Halmos G, Armatis P, Cai R Z, Csernus V, Kovacs M, Koppan M, Szepeshazi K, Kahan Z. Proc Natl Acad Sci USA. 1998;95:1794–1799. doi: 10.1073/pnas.95.4.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egami H, Takiyama Y, Cano M, Houser W H, Pour P M. Carcinogenesis. 1989;10:861–869. doi: 10.1093/carcin/10.5.861. [DOI] [PubMed] [Google Scholar]
- 20.Kumble K D, Hirota M, Pour P M, Vishwanatha J K. Cancer Res. 1992;52:163–167. [PubMed] [Google Scholar]
- 21.Caron P, Buscail L, Beckers A, Esteve J P, Igout A, Hennen G, Susini C. J Clin Endocrinol Metab. 1997;82:3771–3776. doi: 10.1210/jcem.82.11.4350. [DOI] [PubMed] [Google Scholar]
- 22.Egami H, Tomioka T, Tempero M, Kay D, Pour P M. Am J Pathol. 1991;138:557–561. [PMC free article] [PubMed] [Google Scholar]
- 23.Schally A V, Nagy A. Eur J Endocrinol. 1999;141:1–14. doi: 10.1530/eje.0.1410001. [DOI] [PubMed] [Google Scholar]
- 24.Perl A K, Wilgenbus P, Dahl U, Semb H, Christofori G. Nature (London) 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 25.Mareel M, Berx G, Van Roy F, Bracke M. J Cell Biochem. 1996;61:524–530. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C524::AID-JCB5%3E3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 26.Weinel R J, Neumann K, Kisker O, Rosendahl A. Int J Pancreatol. 1996;19:25–30. doi: 10.1007/BF02788372. [DOI] [PubMed] [Google Scholar]
- 27.Soler C, Rousselle P, Damour O. Cell Adhes Commun. 1998;5:13–25. doi: 10.3109/15419069809005595. [DOI] [PubMed] [Google Scholar]
- 28.Owens D W, McLean G W, Wyke A W, Paraskeva C, Parkinson E K, Frame M C, Brunton V G. Mol Biol Cell. 2000;11:51–64. doi: 10.1091/mbc.11.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]