Abstract
An important goal of tissue engineering is to achieve reconstitution of specific functionally active cell types by transplantation of differentiated cell populations derived from normal or genetically altered embryonic stem cells in vitro. We find that mast cells derived in vitro from wild-type or genetically manipulated embryonic stem cells can survive and orchestrate immunologically specific IgE-dependent reactions after transplantation into mast cell-deficient KitW/KitW-v mice. These findings define a unique approach for analyzing the effects of mutations of any genes that are expressed in mast cells, including embryonic lethal mutations, in vitro or in vivo.
Embryonic stem (ES) cells, derived from the inner cell mass of developing blastocysts, can grow continuously in an undifferentiated state in culture but have the potential to develop into all adult tissues and cell types (1–3). Accordingly, ES cells are widely regarded as important vehicles for tissue transplantation and gene therapy (2, 3). One approach to realize the therapeutic potential of ES cells is to generate lineage-committed progenitor cells from ES cells in vitro, and then to transplant such cells so that they can undergo further differentiation in vivo (4–10). In this study, we have developed an alternative approach: to generate from ES cells a differentiated cell type that can express cell lineage-specific function in vitro and then to transplant such differentiated cells to restore such functional activity in vivo.
We used mast cells as the model system for this effort because we and others have shown that functionally active mast cells can be generated in large numbers from appropriate hematopoietic precursor cells in vitro (reviewed in ref. 11) and because of the potential to use mutant mice that essentially lack mast cells (WBB6F1-Kitw/Kitw-v mice, ref. 12) as recipients to test the cell specific-function of adoptively transferred mast cell populations. Mast cells are major effector cells of IgE Ab-associated allergic reactions, and their immunological function in such responses is mediated primarily via the high-affinity IgE receptor, FcɛRI (13–15). Whereas Kitw/Kitw-v mice express no detectable mast cell-dependent immunological activity in vivo, they can express IgE-dependent mast cell function after the adoptive transfer of mast cells derived from hematopoietic precursor cells in vitro (13, 16).
To evaluate the potential versatility of ES cell-derived mast cells (ESMCs) for mechanistic studies of mast cell development and function, we wished to assess whether this approach could be used to generate and analyze both wild-type ESMCs and ESMCs derived from genetically manipulated ES cells, including those that express embryonic lethal mutations. Accordingly, we used wild-type ES cells and ES cells that were null for stress-activated protein kinase (SAPK)/extracellular signal-regulated kinase (ERK) kinase-1 (SEK1, refs. 17–20). SEK1 is a mitogen-activated protein (MAP) kinase kinase that is activated in response to various stress stimuli and certain proinflammatory cytokines (17, 18). SEK1 is also activated in response to FcɛRI crosslinking (21), and SEK1 can regulate c-Jun NH2-terminal protein kinase (JNK) (17,18). Although JNK (22) and therefore SEK1 (21) have been implicated in mast cell tumor necrosis factor (TNF)-α production, the fact that SEK1 −/− is an embryonic lethal genotype (19, 20) made it difficult to assess directly the importance of SEK1 in mast cell development or FcɛRI-dependent activation and secretion of TNF-α.
In this report, we demonstrate that functionally active differentiated mast cells can be generated from normal or SEK1 −/− ES cells in vitro and show that such ESMCs can survive and express IgE-dependent immunological function after transplantation into mast cell-deficient KitW/KitW-v mice.
Materials and Methods
In Vitro Differentiation of Mast Cells from ES Cells.
Strain 129Sv wild-type W9.5 (SEK1 +/+) and SEK1 −/− ES (20) cells were maintained undifferentiated in gelatin-coated dishes in DMEM (GIBCO) containing 15% FBS (HyClone), 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM MEM nonessential amino acids (all GIBCO), 143 μM monothioglycerol (Sigma), and 5 ng/ml recombinant mouse leukemia inhibitory factor (rmLIF, R & D Systems). To form embryoid bodies (EBs), 2000 ES cells were plated in bacterial-grade dishes in 1.5 ml Iscove's modified Dulbecco's medium (IMDM) containing 0.9% methylcellulose (Fluka), 15% FBS, 2 mM l-glutamine, 434 μM monothioglycerol, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.1 mM MEM nonessential amino acids, 50 ng/ml rat stem cell factor (rrSCF, Amgen Biologicals), and 5 ng/ml rmIL-11 (R & D Systems). The cultures were replenished 7 days later with 1 ml of feeding medium containing 60 ng/ml rrSCF, 30 ng/ml rmIL-3, and 30 ng/ml rmIL-6. Mast cell differentiation was induced by transferring EBs to tissue culture grade dishes in liquid medium (DMEM, 10% FCS/2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin/20% vol/vol WEHI-3 cell-conditioned medium/50 ng/ml rrSCF) 2 wk after primary plating. Cultures were then expanded weekly by transferring the cells to new flasks and replacing half of the medium.
Generation of Bone Marrow-Derived Cultured Mast Cells (BMCMCs).
BMCMCs were obtained by maintaining the femoral bone marrow cells of 4- to 6-wk-old 129Sv mice in liquid medium containing IL-3 and SCF as described above for in vitro differentiation of mast cells from EBs. Cultures underwent replacement of half of the medium weekly. After 4–5 wk, at least 95% of the cells that remained in the cultures were identifiable as mast cells as determined by May-Grünwald/Giemsa staining (23).
Histochemical Staining.
For assessment of morphological and histochemical characteristics, cytocentrifuged preparations of ESMCs and BMCMCs were stained with Wright Giemsa (Fisher) according to the manufacturer's instruction or with Alcian blue/Safranin as previously described (24). To assess another aspect of the phenotypic maturation of BMCMCs or ESMCs, the cells were stained with 0.02% Berberine Sulfate (Sigma), a yellow fluorescent dye that binds to the heparin in mature mast cell granules (24), for 20 min at room temperature, rinsed in distilled deionized H20 (ddH2O) (pH 4.0) and observed under a fluorescent microscope.
Reverse Transcriptase–PCR Analysis of Mast Cell Proteases.
RNA was extracted from either ESMCs or BMCMCs of 129Sv mouse origin by TRI REAGENT (Molecular Research Center, Cincinnati) according to the manufacturer's specifications at various time points after differentiation. Mouse mast cell-associated protease (mMCP)-2, mMCP-4, mouse mast cell carboxypeptidase A (mMC-CPA), and β-actin mRNA expression was analyzed by reverse transcriptase-PCR as previously described (25).
Flow Cytometry.
Expression of the high-affinity IgE receptor, FcɛRI, and the SCF receptor, c-kit, was analyzed by flow cytometry as described (26) with slight modification. Briefly, ESMCs, BMCMCs, or peritoneal cells isolated from WBB6F1-+/+ (Kit+/+) mice or KitW/KitW-v mice reconstituted i.p. with ESMCs were first preincubated with 10 μg/ml of the anti-CD23 B3B4 mAb (PharMingen) and 10 μg/ml of the anti-FcγRII/III 2.4G2 mAb (PharMingen) for 15 min to block low-affinity binding of IgE or other subsequent Abs to CD23+ or FcγRII/III+ cells, respectively. Cells were then incubated with 5 μg/ml mouse IgE anti-dinitrophenol (DNP) mAb (Sigma) for 45 min, followed by a simultaneous incubation of 15 μg/ml of biotinylated-rat-anti-mouse c-kit Ab (PharMingen) for 25 min. After washing, cells were stained with 10 μg/ml FITC-rat-anti-mouse IgE Ab (PharMingen) and 1 μg/ml phycoerythrin-streptavidin (PharMingen) for 25 min. All steps were performed at 4°C in DMEM containing 2% FCS (Sigma). Amounts equal to 10,000 stained cells in each sample were analyzed by using a FACSCalibur (Becton Dickinson). An instrument setting of the flow cytometer that was appropriate for analysis of peritoneal cells was determined in the first experiment and was used for all such analysis, whereas a modified instrument setting was used for the analysis of cultured cells. The flow cytometry setting for ESMCs and BMCMCs was not changed throughout this study.
Measurement of Cytokine and Mediator Release.
To activate mast cells via FcɛRI stimulation, ESMCs or BMCMCs were first sensitized at 37°C with anti-DNP-human serum albumin (HSA) IgE (10 μg/ml) for 2 h and then stimulated with DNP-HSA (Sigma) Ag. To measure the release of β-hexosaminidase, sensitized cells were stimulated for 1 h with DNP-HSA (10 ng/ml) or with the calcium-ionophore, ionomycin (1 μM, Sigma) in Tyrode's buffer, or vehicle alone. Cell supernatants were harvested, and cell pellets were lysed with 0.5% Triton X (Sigma) in Tyrode's buffer. β-hexosaminidase was quantified in the supernatants and pellet lysates by spectrophotometric analysis of hydrolysis of p-NAG (p-nitrophenyl-N-acetyl-β-d-glucosaminidine) (27). β-hexosaminidase release was calculated as the percentage of β-hexosaminidase present in the supernatants. To measure the release of cytokines, IgE-sensitized cells were stimulated for 6 h in the presence or absence of DNP-HSA (23). TNF-α and IL-6 levels in the supernatants were measured by ELISA Kits (R&D systems) according to the manufacturer's instructions.
Mast Cell Reconstitution and Induction of Passive Cutaneous Anaphylaxis (PCA).
Genetically mast cell-deficient mice and the congenic normal (+/+) mice (WB/ReJ-KitW/+ × C57BL/6J-KitW-v/+)F1-(KitW/KitW-v, Kit+/+), designated here WBB6F1-(KitW/KitW-v, Kit+/+), were purchased from The Jackson Laboratory. The skin of adult WBB6F1-KitW/KitW-v mice contains <1% the number of mast cells present in the skin of the congenic (+/+) mice, and KitW/KitW-v mice contain no mast cells in the peritoneal cavity or multiple other anatomical sites (12). For studies of mast cell survival in vivo, mast cell-deficient KitW/KitW-v mice were injected i.p. with 2 × 106 BMCMCs or SEK1 +/+ or SEK1 −/− ESMCs. Cells for injection were prepared in 200 μl of HMEM-pipes [Hanks' MEM containing 0.47 g/liter piperazine-N,N′ bis(2-ethane sulfonic acid)]. The percentage of mast cells in the peritoneal lavage was determined by Kimura staining (28) 3–4 wk or 8 wk after peritoneal reconstitution. For studies of the ability of ESMCs to elicit immunologically specific reactions in vivo, 1 × 106 SEK1 +/+ or SEK1 −/− ESMCs, in 20 μl of HMEM-pipes, were injected intradermally into the ears of KitW/KitW-v mice. To elicit PCA reactions 3 wk later (16), mice were first primed with IgE by the intradermal injection, into the ears, of 100 ng monoclonal mouse dinitrophenol-specific (anti-DNP) IgE in 20 μl of HMEM-pipes; control ears were injected with vehicle alone. Twenty-four hours later, the mice were injected i.v. with 200 μg of Ag (DNP-HSA) diluted in sterile 0.9% NaCl. Ear swelling was quantified by three consecutive measurements of ear thickness by using a Mitutoyo (Aurora, IL) engineer's micrometer before (baseline) and 1, 2, 4, and 6 h after Ag challenge. The use and care of animals in this study was in accord with the guidelines of the National Institutes of Health and the Stanford University Committee on Animal Welfare.
Results and Discussion
We first modified the two-step replating assay of Keller et al. (29, 30) to identify conditions that could permit the generation of large quantities of highly purified ESMCs. Instead of replating disaggregated EBs into methylcellulose medium, which results in the differentiation of mast cells and other hematopoietic lineages, but in very limited numbers (30), we placed the EBs in tissue culture dishes containing liquid medium supplemented with SCF and/or IL-3 (31), which allowed the EBs to attach and spread on the dishes and give rise to mast cells. After extensive preliminary studies, we found that the highest yields of mast cells were obtained in medium containing both SCF and IL-3; e.g., by ≈6 wk after EB differentiation, we typically generate ≈108 ESMCs from 2,000 ES cells, a 50,000-fold expansion.
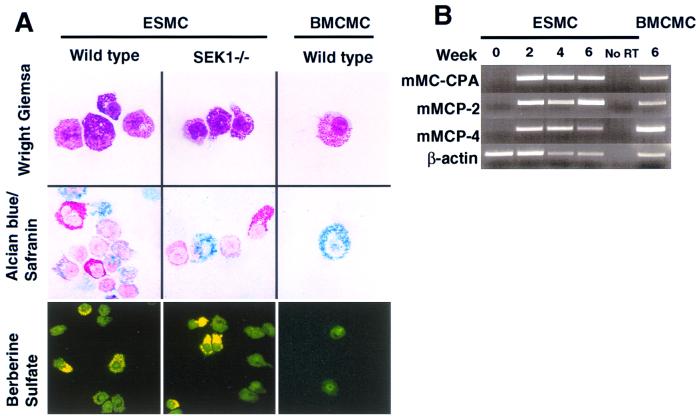
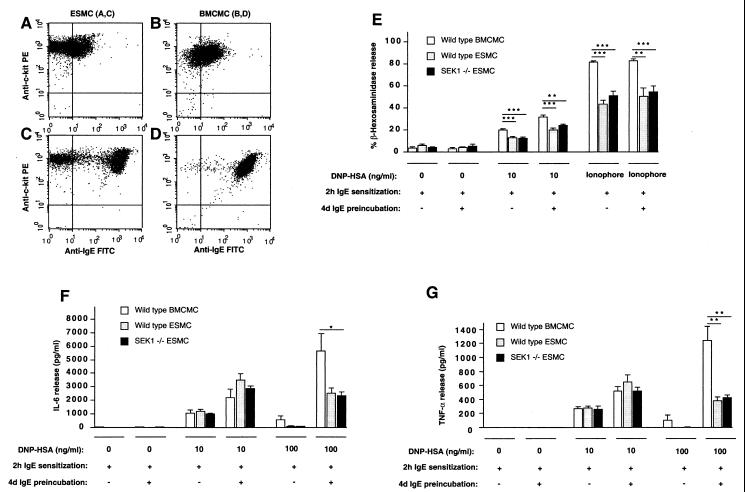
ESMCs exhibited morphological and cytochemical characteristics of mouse mast cells (24, 32) (Fig. 1A) and expressed mRNA for mouse mast cell-specific proteases (33, 34) (Fig. 1B). Compared with adult mouse BMCMCs that were generated in the identical liquid medium, ESMCs expressed similar levels of c-kit (cf. Fig. 2 A vs. B) and IgE-dependent up-regulation of surface expression of the receptor that binds IgE Abs with high-affinity (FcɛRI) (cf. Fig. 2 C vs. D) (26). However, the ESMCs differed from BMCMCs in that ESMCs (i) exhibited stronger reactivity with Berberine Sulfate (indicative of the presence of heparin; ref. 24) and Safranin (Fig. 1A) and thus, by these criteria, more closely resembled so-called “connective tissue-type mast cells” than did the BMCMCs (reviewed in refs. 11, 24, and 32) and (ii) contained a higher proportion of c-kit+ FcɛRI− mast cells (cf. Fig. 2 C vs. D).
Figure 1.
Phenotypic characteristics of ESMCs or BMCMCs of 129Sv mouse origin were generated by culturing EBs or bone marrow, respectively, for 4 wk in liquid medium containing 20% WEHI-3 cell-conditioned medium (as a source of IL-3) supplemented with 50 ng/ml rrSCF (WEHI + SCF). (A) Cytocentrifuged preparations of wild-type or SEK1 −/− ESMCs, or wild-type BMCMCs, were stained with Wright Giemsa, Alcian blue/Safranin, or Berberine Sulfate. (B) Reverse transcriptase-PCR analysis of mMCP-2, mMCP-4, and mouse mast cell carboxypeptidase A (mMC-CPA) gene expression in wild-type ES cells before (0) or after placing the cells in WEHI + SCF for 2, 4, or 6 wk, or in BMCMCs 6 wk after differentiation in WEHI + SCF. No RT = no reverse transcriptase.
Figure 2.
ESMCs express functionally active FcɛRI receptors. (A–D) Flow cytometry analysis (104 cells/condition) of c-kit and FcɛRI expression in wild-type (129Sv) ESMCs (A and C) and BMCMCs (B and D) with (C and D) or without (A and B) preincubation with IgE (5 μg/ml) for 4 days (ref. 26). (E–G) Release of (E) β-hexosaminidase, (F) IL-6, or (G) TNF-α in wild-type BMCMCs, wild-type ESMCs, or SEK1 −/− ESMCs stimulated with IgE and specific Ag (DNP-HSA) (E–G) or calcium ionophore (E). Before stimulation, some cells (+) were preincubated with anti-DNP-HSA IgE (5 μg/ml) for 4 days to enhance FcɛRI expression. All cells were then sensitized with anti-DNP-HSA IgE (10 μg/ml) for 2 h and then stimulated with DNP-HSA or calcium ionophore. The percentage of of β-hexosaminidase release and cytokine release (23) were measured 1 or 6 h after Ag stimulation, respectively. In E–G, the results are mean ± SEM (n = 4–10) and are representative of the results obtained in two to three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005, as compared with values for BMCMCs by unpaired Student's t test.
We found that ESMCs could be generated from SEK1 −/− or wild-type ES cells with similar efficiency and that SEK1 −/− and wild-type ESMCs exhibited similar morphological and cytochemical characteristics (Fig. 1A) and similar patterns of expression of surface c-kit and FcɛRI and mRNA for mMCP-2, mMCP-4, and mouse mast cell carboxypeptidase A (mMC-CPA) (data not shown).
Wild-type and SEK1 −/− ESMCs exhibited statistically indistinguishable levels of release of the preformed mediator β-hexosaminidase (Fig. 2E), as well as the cytokines IL-6 (Fig. 2F) and TNF-α (Fig. 2G), after IgE- and Ag (DNP-HSA)-dependent activation or after stimulation with calcium ionophore (Fig. 2E). These data indicate that SEK1 is not required for FcɛRI-dependent mast cell degranulation and cytokine production in vitro. In accord with our previous findings in BMCMCs (26), higher levels of IgE-dependent β-hexosaminidase and cytokine release were observed in ESMCs that had undergone up-regulation of FcɛRI expression as a result of a 4-day preincubation with IgE Ab before further sensitization with IgE and Ag challenge (Fig. 2 E–G). Under certain conditions of stimulation, β-hexosaminidase and cytokine release were greater in BMCMCs than in ESMCs. It will be of interest to investigate the reason(s) for this difference. ESMCs contained higher fractions of FcɛRI− cells than did BMCMCs (e.g., cf. Fig. 2 A and C with Fig. 2 B and D), but this difference may not explain the lower levels of responsiveness in ESMCs, because these cells also released less β-hexosaminidase than did BMCMCs after FcɛRI-independent stimulation with ionomycin (Fig. 2E).
We next investigated the ability of ESMCs to survive and express immunologically specific function after transfer into genetically mast cell-deficient KitW/KitW-v mice in vivo. In contrast to the congenic normal Kit+/+ mice, in which ≈3%–4% of the cells in peritoneal lavage fluid are mast cells (black bar in Fig. 3A), no mast cells are ordinarily present in the peritoneal cavity or many other tissues of KitW/KitW-v mice (11, 12). SEK1 −/− or wild-type ESMCs survived in the peritoneal cavities of KitW/KitW-v mice for 8 wk (Fig. 3A) and exhibited even stronger reactivity with Berberine Sulfate than did ESMCs examined at the time of i.p. injection (data not shown). Mice injected with SEK1 +/+ ESMCs had fewer peritoneal mast cells at 3 wk than did mice injected with either SEK1 −/− ESMCs or BMCMCs. However, by 8 wk, there were no significant differences in the percentage of peritoneal mast cells among any of the groups analyzed (Fig. 3A). Moreover, the ESMCs recovered from these animals (Fig. 3B) expressed surface levels of c-kit and FcɛRI that were similar to those observed in the native peritoneal mast cells of Kit+/+ normal mice (Fig. 3C).
Figure 3.
Survival of ESMCs in vivo. (A) Mast cell-deficient WBB6F1-KitW/KitW-v mice (n = 6–15/group, 8–16 wk old) were injected i.p. with 2 × 106 SEK1 +/+ or SEK1 −/− ESMCs, or BMCMCs of WBB6F1-+/+ (Kit+/+) origin. At 3–4 or 8 wk after injections, peritoneal lavage was performed, and the percentage of mast cells present in the lavage was evaluated by Kimura staining. The percentage of native peritoneal mast cells (PMCs) in WBB6F1-+/+ (Kit+/+) mice was counted as the positive control (black bar). *, P < 0.05 as compared with values for mice at 3 wk after injection of SEK1 −/− ESMCs or BMCMCs. (B and C) Flow cytometry analysis of c-kit and FcɛRI expression on the ESMCs recovered from the peritoneal cavities of WBB6F1-KitW/KitW-v mice 8 wk after reconstitution (B) or on PMCs recovered from Kit+/+ mice (C); mast cells are indicated by the oval.
These results show that ESMCs of 129Sv origin can survive and maintain wild-type levels of mast cell reconstitution for at least 8 wk after injection into the peritoneal cavity of WBB6F1-KitW/KitW-v mice. Whereas strains 129Sv and C57BL/6 (one of the parental strains of WBB6F1 mice) are MHC identical (H-2b), 129Sv and C57BL/6 mice differ at minor histocompatibility loci (35). Accordingly, it will be of interest to attempt to generate ESMCs of C57BL/6 or perhaps even WBB6F1 origin, and then to compare their ability to achieve long-term local or systemic reconstitution of mast cell populations in KitW/KitW-v mice to that of ESMCs of 129Sv origin.
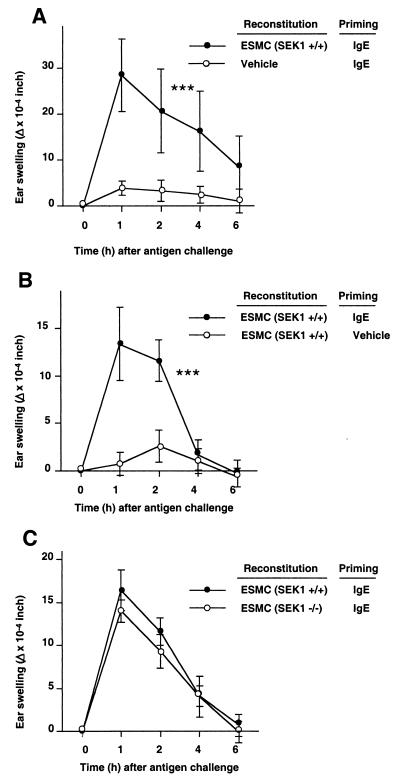
We also assessed whether ESMCs of 129Sv origin could orchestrate a typical IgE- and mast cell-dependent immunological response 3 wk after injection into the ears of KitW/KitW-v mice in vivo. Significant reactions occurred at sites reconstituted with ESMCs and primed with IgE (Fig. 4A), but not at sites that received either ESMCs or IgE alone (Fig. 4 A and B). Moreover, there were no significant differences between the responses detected in KitW/KitW-v mice that had been reconstituted with SEK1 −/− as opposed to SEK1 +/+ ESMCs (Fig. 4C). These data thus support our in vitro findings in excluding an essential role for SEK1 in the expression of important aspects of IgE-dependent mast cell function in vitro or in their ability to orchestrate the tissue swelling associated with IgE-associated PCA reactions in vivo.
Figure 4.
IgE-dependent PCA in mast cell-deficient WBB6F1-KitW/KitW-v mice reconstituted with ESMCs in one or both ears. PCA reactions were induced by challenging i.v. with DNP-HSA Ag (200 μg) 24 h after intradermal injection of anti-DNP-HSA IgE (100 ng) or vehicle into the ears (Priming); the reactions were assessed by measuring ear swelling (Δ = difference in ear thickness before and after Ag challenge). (A) SEK1 +/+ ESMCs in the left ears and vehicle in the right ears (mean ± SEM, n = 5/group). ***, P < 0.0001 vs. vehicle “reconstitution” by ANOVA. (B) SEK1 +/+ ESMCs in both ears, with IgE priming of only the left ears (mean ± SEM, n = 7/group). ***, P < 0.0001 vs. vehicle “priming” by ANOVA. Data representative of the results obtained in three similar experiments. (C) SEK1 +/+ ESMCs in the left ears and SEK1 −/− ESMCs in the right ears. The results (mean ± SEM, n = 7/group) are representative of those obtained in three similar experiments.
In summary, we have demonstrated that a functionally active differentiated hematopoietic cell type not only can be derived from ES cells in vitro but can survive and mediate a typical immunologically specific reaction after adoptive transfer in vivo. Our study also provides proof of principle that one can generate large numbers of a differentiated, functionally active cell type from genetically altered ES cells (including those that contain “embryonic lethal” mutations) in vitro, and then transplant such cells to assess whether these genetic manipulations influence the function of that cell type in vivo. We expect that this approach will be useful for analyzing the mechanisms that regulate mast cell development from hematopoietic precursors. Indeed, even when specific mutations in ES cells interfere with either mast cell differentiation itself, or the generation of sufficient mast cells for in vivo studies, attempts to derive mast cells from such ES cells in vitro may greatly facilitate the identification of gene products that importantly contribute to mast cell development. Our findings also define a new approach for analyzing how mast cells express their function, as well as for investigating the roles of specific gene products (or agents that enhance or suppress their activity) in host defense, tissue remodeling, and models of asthma and other diseases.
Acknowledgments
We thank Gordon Keller and Irving Weissman for their critical reviews of the manuscript. This work was supported by U.S. Public Health Service Grants AI 23990, CA 72074, and AI 41995 (to S.J.G.) and by Deutsche Forschungsgemeinschaft Grant WE 2300/1 (to J.W.).
Abbreviations
- ES cells
embryonic stem cells
- SEK1
stress-activated protein kinase/extracellular signal-regulated kinase (SAPK/ERK) kinase-1
- EBs
embryoid bodies
- SCF
stem cell factor
- ESMCs
ES cell-derived mast cells
- BMCMCs
bone marrow-derived cultured mast cells
- DNP-HSA
dinitrophenol-human serum albumin
- TNF
tumor necrosis factor
- mMCP
mouse mast cell-associated protease
- PCA
passive cutaneous anaphylaxis
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.160254997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.160254997
References
- 1.Robertson E J. Terotocarcinomas and Embryonic Stem Cells: A Practical Approach. Oxford: IRL; 1987. [Google Scholar]
- 2.Gearhart J. Science. 1998;282:1061–1062. doi: 10.1126/science.282.5391.1061. [DOI] [PubMed] [Google Scholar]
- 3.Keller G, Snodgrass H R. Nat Med. 1999;5:151–152. doi: 10.1038/5512. [DOI] [PubMed] [Google Scholar]
- 4.Weiss M J, Orkin S H. J Clin Invest. 1996;97:591–595. doi: 10.1172/JCI118454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klug M G, Soonpaa M H, Koh G Y, Field L J. J Clin Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deacon T, Dinsmore J, Costantini L C, Ratliff J, Isacson O. Exp Neurol. 1998;149:28–41. doi: 10.1006/exnr.1997.6674. [DOI] [PubMed] [Google Scholar]
- 7.McDonald J W, Liu X-Z, Qu Y, Liu S, Mickey S K, Turetsky D, Gottlieb D I, Choi D W. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 8.Brüstle O, Jones K N, Learish R D, Karram K, Choudhary K, Wiestler O D, Duncan I D, McKay R D G. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez-Ramos J C, Palacios R. Proc Natl Acad Sci USA. 1992;89:9171–9175. doi: 10.1073/pnas.89.19.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potocnik A J, Kohler H, Eichmann K. Proc Natl Acad Sci USA. 1997;94:10295–10300. doi: 10.1073/pnas.94.19.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galli S J, Zsebo K M, Geissler E N. Adv Immunol. 1994;55:1–96. doi: 10.1016/s0065-2776(08)60508-8. [DOI] [PubMed] [Google Scholar]
- 12.Kitamura Y, Go S, Hatanaka K. Blood. 1978;52:447–452. [PubMed] [Google Scholar]
- 13.Galli S J. N Engl J Med. 1993;328:257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 14.Beaven M A, Metzger H. Immunol Today. 1993;14:222–226. doi: 10.1016/0167-5699(93)90167-j. [DOI] [PubMed] [Google Scholar]
- 15.Kinet J-P. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 16.Wershil B K, Mekori Y A, Murakami T, Galli S J. J Immunol. 1987;139:2605–2614. [PubMed] [Google Scholar]
- 17.Sanchez I, Hughes R T, Mayer B J, Yee K, Woodgett J, R, Avruch J, Kyriakis J M, Zon L I. Nature (London) 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- 18.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Nature (London) 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 19.Yang D, Tournier C, Wysk M, Lu H-T, Xu J, Davis R J, Flavell R A. Proc Natl Acad Sci USA. 1997;94:3004–3009. doi: 10.1073/pnas.94.7.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganiatsas S, Kwee L, Fujiwara Y, Perkins A, Ikeda T, Labow M A, Zon L I. Proc Natl Acad Sci USA. 1998;95:6881–6886. doi: 10.1073/pnas.95.12.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawakami Y, Hartman S E, Holland P M, Cooper J A, Kawakami T. J Immunol. 1998;161:1795–1802. [PubMed] [Google Scholar]
- 22.Ishizuka T, Terada N, Gerwins P, Hamelmann E, Oshiba A, Fanger G R, Johnson G L, Gelfand E W. Proc Natl Acad Sci USA. 1997;94:6358–6363. doi: 10.1073/pnas.94.12.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagari E, Tsai M, Lantz C S, Fox L G, Galli S J. Blood. 1997;89:2654–2663. [PubMed] [Google Scholar]
- 24.Tsai M, Takeishi T, Thompson H, Langley K E, Zsebo K M, Metcalfe D D, Geissler E N, Galli S J. Proc Natl Acad Sci USA. 1991;88:6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai M, Miyamoto M, Tam S-Y, Wang Z-S, Galli S J. Am J Pathol. 1995;146:335–343. [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi M, Lantz C S, Oettgen H C, Katona I M, Fleming T, Miyajima I, Kinet J-P, Galli S J. J Exp Med. 1997;185:663–672. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortega E, Hazan B, Zor O, Pecht I. Eur J Immunol. 1989;19:2251–2256. doi: 10.1002/eji.1830191211. [DOI] [PubMed] [Google Scholar]
- 28.Kimura I, Moritani Y, Tanizaki Y. Clin Allergy. 1973;3:195–202. doi: 10.1111/j.1365-2222.1973.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 29.Wiles M V, Keller G. Development. 1991;111:259–267. doi: 10.1242/dev.111.2.259. [DOI] [PubMed] [Google Scholar]
- 30.Keller G, Kennedy M, Papayannopoulou T, Wiles M V. Mol Cell Biol. 1993;13:473–486. doi: 10.1128/mcb.13.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lantz C S, Boesiger J, Song C H, Mach N, Kobayashi T, Mulligan R C, Nawa Y, Dranoff G, Galli S J. Nature (London) 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 32.Tsai M, Shih L-S, Newlands G F J, Takeishi T, Langley K E, Zsebo K M, Miller H R P, Geissler E N, Galli S J. J Exp Med. 1991;174:125–131. doi: 10.1084/jem.174.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang C, Sali A, Stevens R L. J Clin Immunol. 1998;18:169–183. doi: 10.1023/a:1020574820797. [DOI] [PubMed] [Google Scholar]
- 34.Rodewald H-R, Dessing M, Dvorak A M, Galli S J. Science. 1996;271:818–822. doi: 10.1126/science.271.5250.818. [DOI] [PubMed] [Google Scholar]
- 35.Russell E S. Adv Genetics. 1979;20:357–459. [PubMed] [Google Scholar]