Abstract
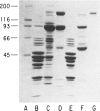
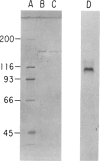
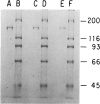
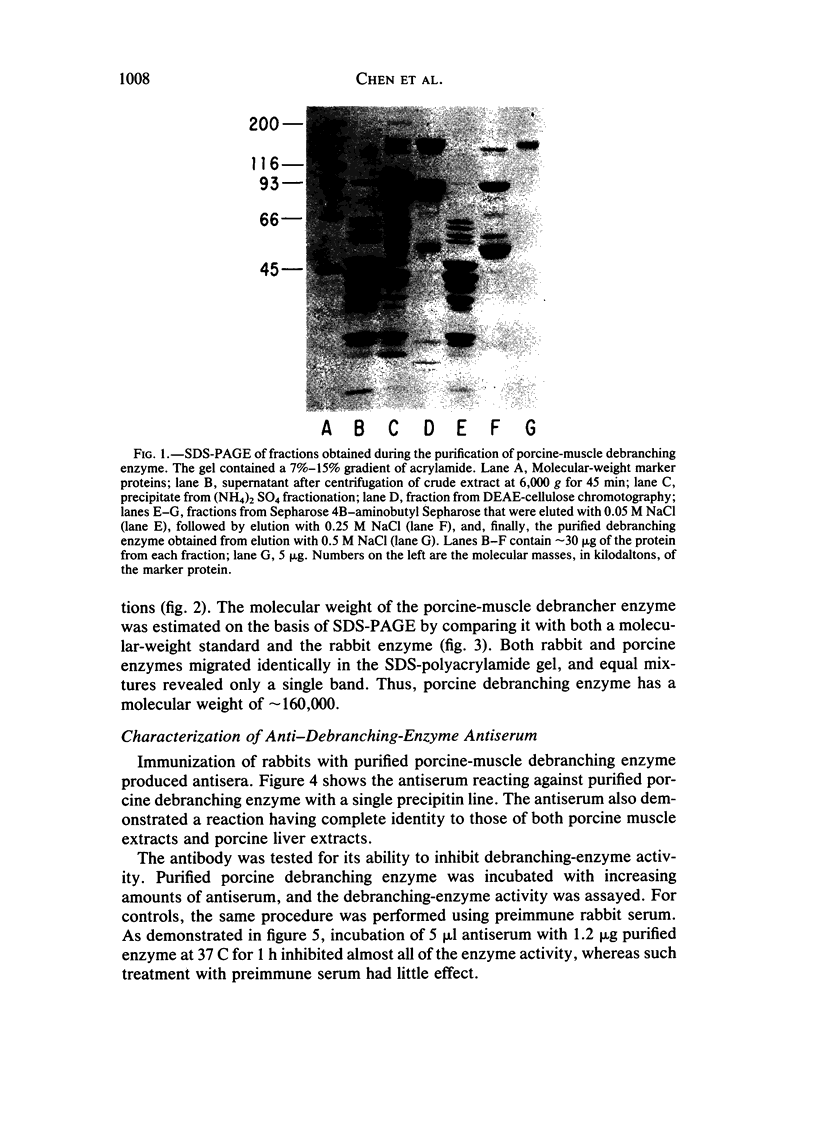
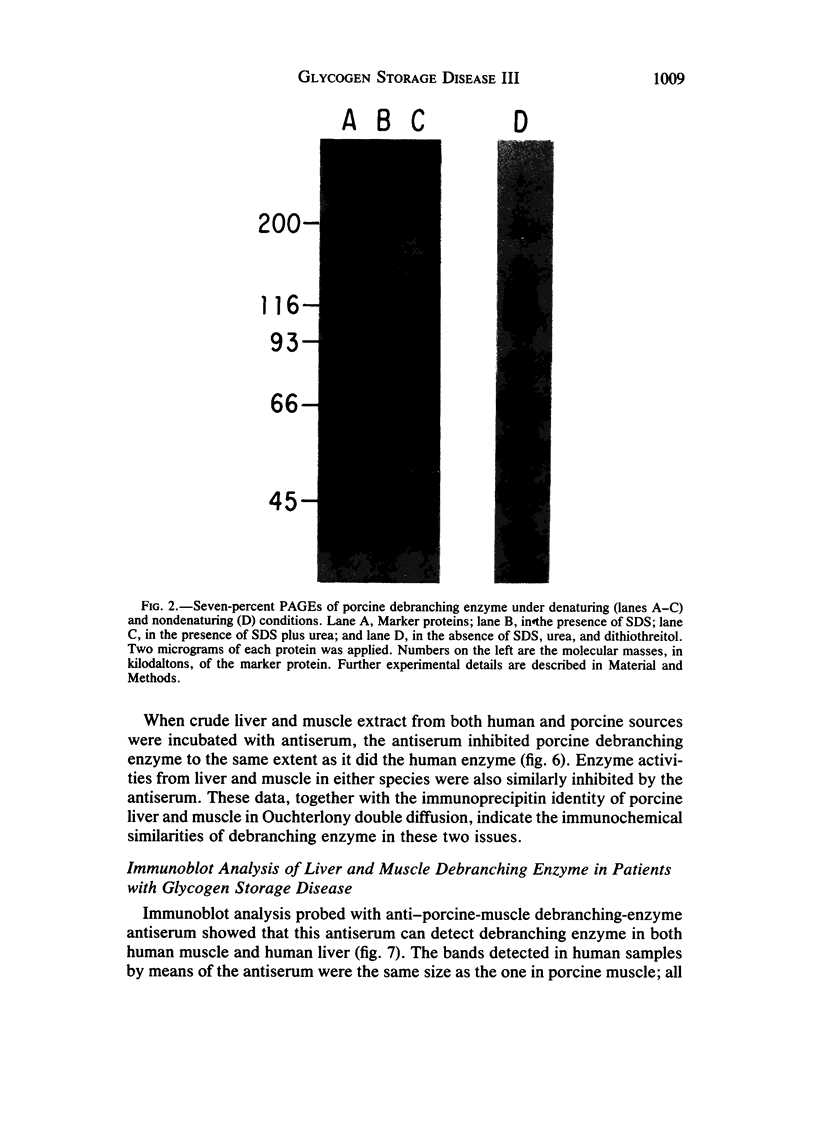
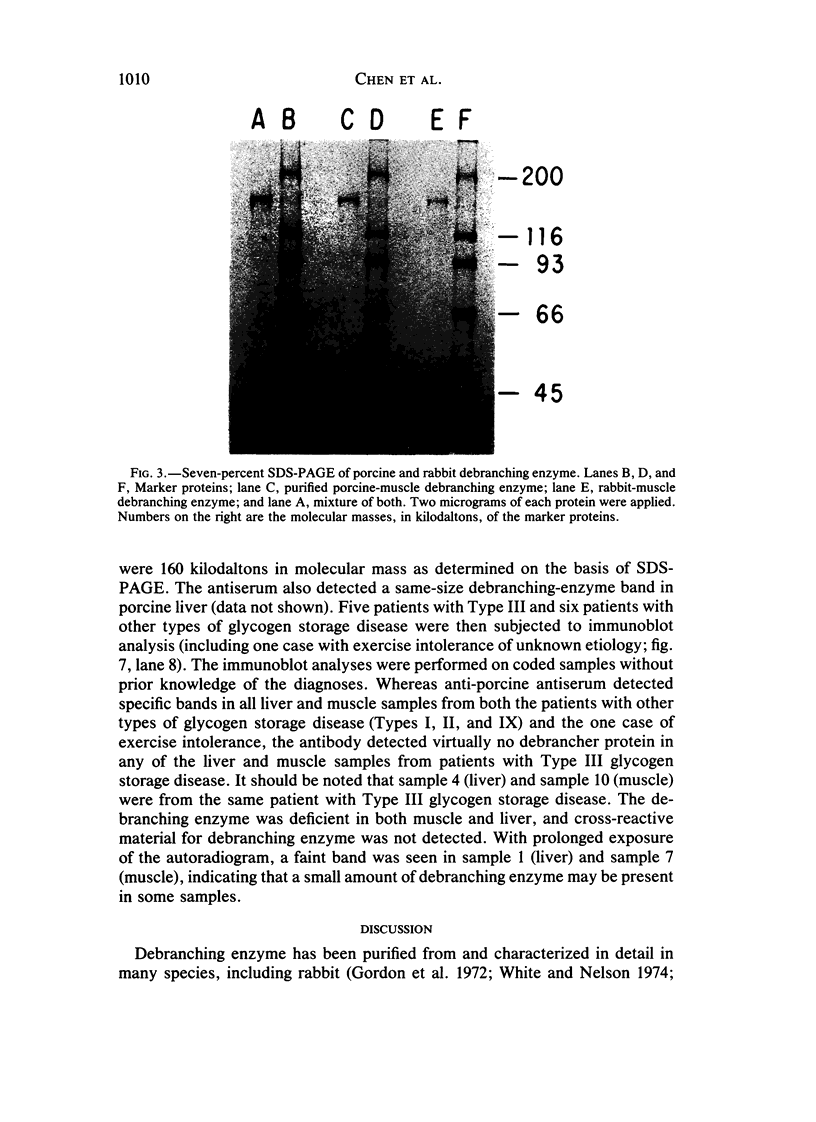
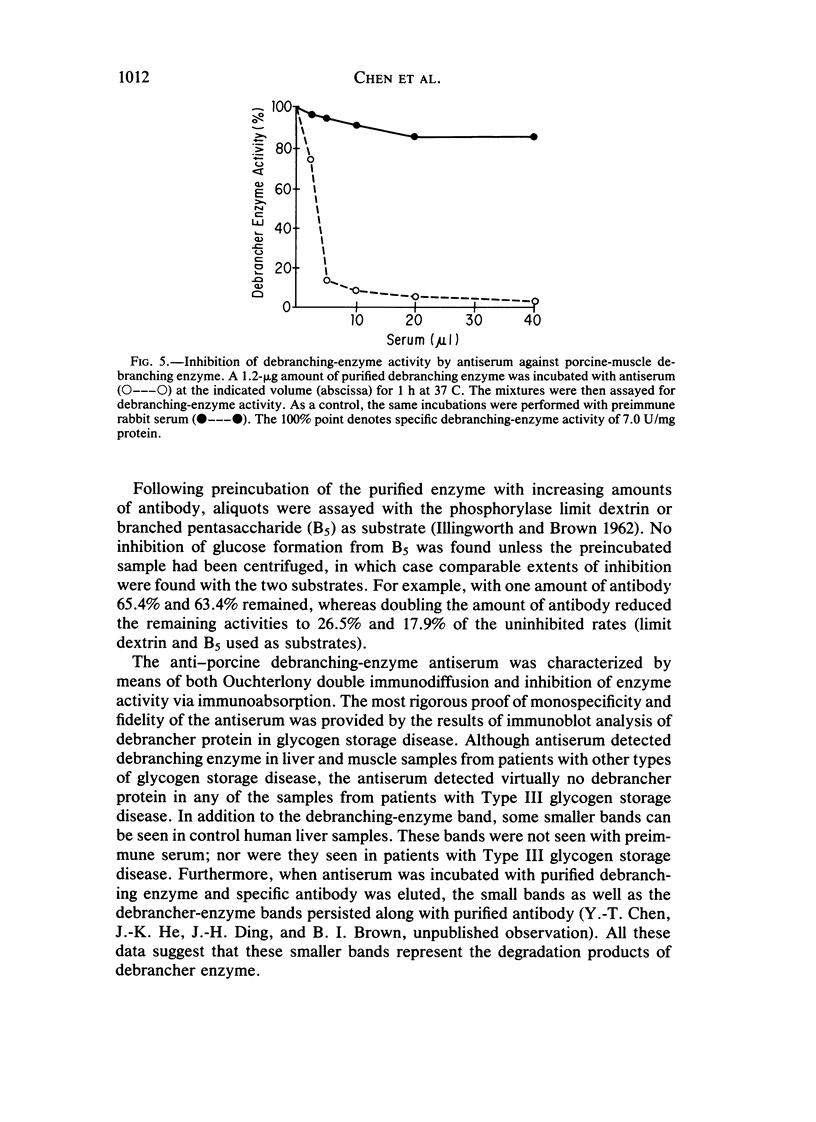
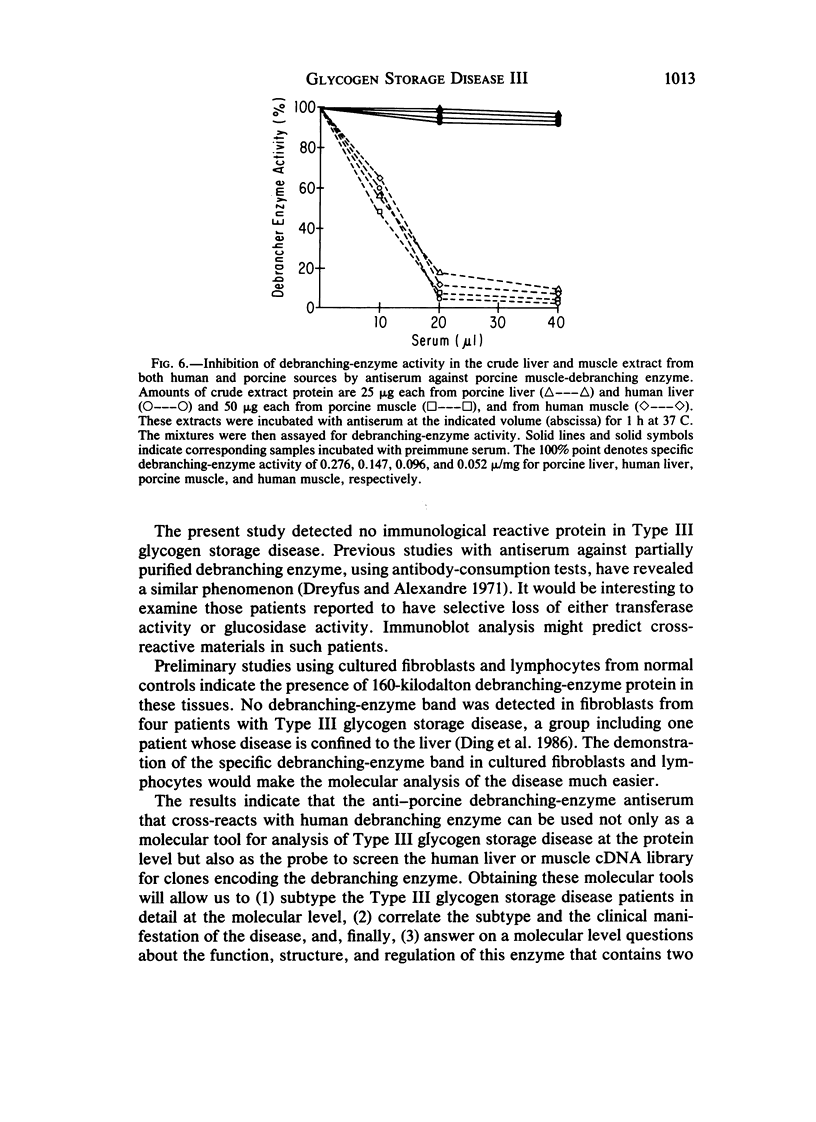
Type III glycogen storage disease is caused by a deficiency of glycogen debranching-enzyme activity. Many patients with this disease have both liver and muscle involvement, whereas others have only liver involvement without clinical or laboratory evidence of myopathy. To improve our understanding of the molecular basis of the disease, debranching enzyme was purified 238-fold from porcine skeletal muscle. In sodium dodecyl sulfate-polyacrylamide gel electrophoresis the purified enzyme gave a single band with a relative molecular weight of 160,000 that migrated to the same position as purified rabbit-muscle debranching enzyme. Antiserum against porcine debranching enzyme was prepared in rabbit. The antiserum reacted against porcine debranching enzyme with a single precipitin line and demonstrated a reaction having complete identity to those of both the enzyme present in crude muscle and the enzyme present in liver extracts. Incubation of antiserum with purified porcine debranching enzyme inhibited almost all enzyme activity, whereas such treatment with preimmune serum had little effect. The antiserum also inhibited debranching-enzyme activity in crude liver extracts from both pigs and humans to the same extent as was observed in muscle. Immunoblot analysis probed with anti-porcine-muscle debranching-enzyme antiserum showed that the antiserum can detect debranching enzyme in both human muscle and human liver. The bands detected in human samples by the antiserum were the same size as the one detected in porcine muscle. Five patients with Type III and six patients with other types of glycogen storage disease were subjected to immunoblot analysis. Although anti-porcine antiserum detected specific bands in all liver and muscle samples from patients with other types of glycogen storage disease (Types I, II, and IX), the antiserum detected no cross-reactive material in any of the liver or muscle samples from patients with Type III glycogen storage disease. These data indicate (1) immunochemical similarity of debranching enzyme in liver and muscle and (2) that deficiency of debranching-enzyme activity in Type III glycogen storage disease is due to absence of debrancher protein in the patients that we studied.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates E. J., Heaton G. M., Taylor C., Kernohan J. C., Cohen P. Debranching enzyme from rabbit skeletal muscle; evidence for the location of two active centres on a single polypeptide chain. FEBS Lett. 1975 Oct 15;58(1):181–185. doi: 10.1016/0014-5793(75)80254-7. [DOI] [PubMed] [Google Scholar]
- Becker J. U., Long T. J., Fischer E. H. Purification and properties of debranching enzyme from dogfish muscle. Biochemistry. 1977 Jan 25;16(2):291–297. doi: 10.1021/bi00621a021. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dreyfus J. C., Alexandre Y. Immunological studies on glycogen storage diseases type 3 and V. Demonstration of the presence of an immunoreactive protein in one case of muscle phosphorylase deficiency. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1364–1370. doi: 10.1016/s0006-291x(71)80236-x. [DOI] [PubMed] [Google Scholar]
- Gillard B. K., Nelson T. E. Amylo-1,6-glucosidase/4-alpha-glucanotransferase: use of reversible substrate model inhibitors to study the binding and active sites of rabbit muscle debranching enzyme. Biochemistry. 1977 Sep 6;16(18):3978–3987. doi: 10.1021/bi00637a007. [DOI] [PubMed] [Google Scholar]
- Gordon R. B., Brown D. H., Brown B. I. Preparation and properties of the glycogen-debranching enzyme from rabbit liver. Biochim Biophys Acta. 1972 Nov 10;289(1):97–107. doi: 10.1016/0005-2744(72)90112-x. [DOI] [PubMed] [Google Scholar]
- Illingworth B., Brown D. H. ACTION OF AMYLO-1,6-GLUCOSIDASE ON LOW MOLECULAR WEIGHT SUBSTRATES AND THE ASSAY OF THIS ENZYME IN GLYCOGEN STORAGE DISEASE. Proc Natl Acad Sci U S A. 1962 Sep;48(9):1619–1623. doi: 10.1073/pnas.48.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Carter J. H., Nielsen L. D., Fischer E. H. Purification and properties of yeast amylo-1,6-glucosidase--oligo-1,4 leads to 1,4-glucantransferase. Biochemistry. 1970 May 26;9(11):2347–2355. doi: 10.1021/bi00813a019. [DOI] [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C., Cox A. J., Kernohan J. C., Cohen P. Debranching enzyme from rabbit skeletal muscle. Purification, properties and physiological role. Eur J Biochem. 1975 Feb 3;51(1):105–115. doi: 10.1111/j.1432-1033.1975.tb03911.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof F., Hers H. G. The subgroups of type 3 glycogenosis. Eur J Biochem. 1967 Oct;2(3):265–270. doi: 10.1111/j.1432-1033.1967.tb00134.x. [DOI] [PubMed] [Google Scholar]
- White R. C., Nelson T. E. Re-evaluation of the subunit structure and molecular weight of rabbit muscle amylo-1,6-glucosidase-4-alpha-glucanotransferase. Biochim Biophys Acta. 1974 Sep 13;365(1):274–280. doi: 10.1016/0005-2795(74)90271-2. [DOI] [PubMed] [Google Scholar]
- White R. C., Ruff C. J., Nelson T. E. Purification of glycogen debranching enzyme from rabbit muscle using omega-aminoalkyl agarose chromatography. Anal Biochem. 1981 Aug;115(2):388–390. doi: 10.1016/0003-2697(81)90022-1. [DOI] [PubMed] [Google Scholar]