Abstract
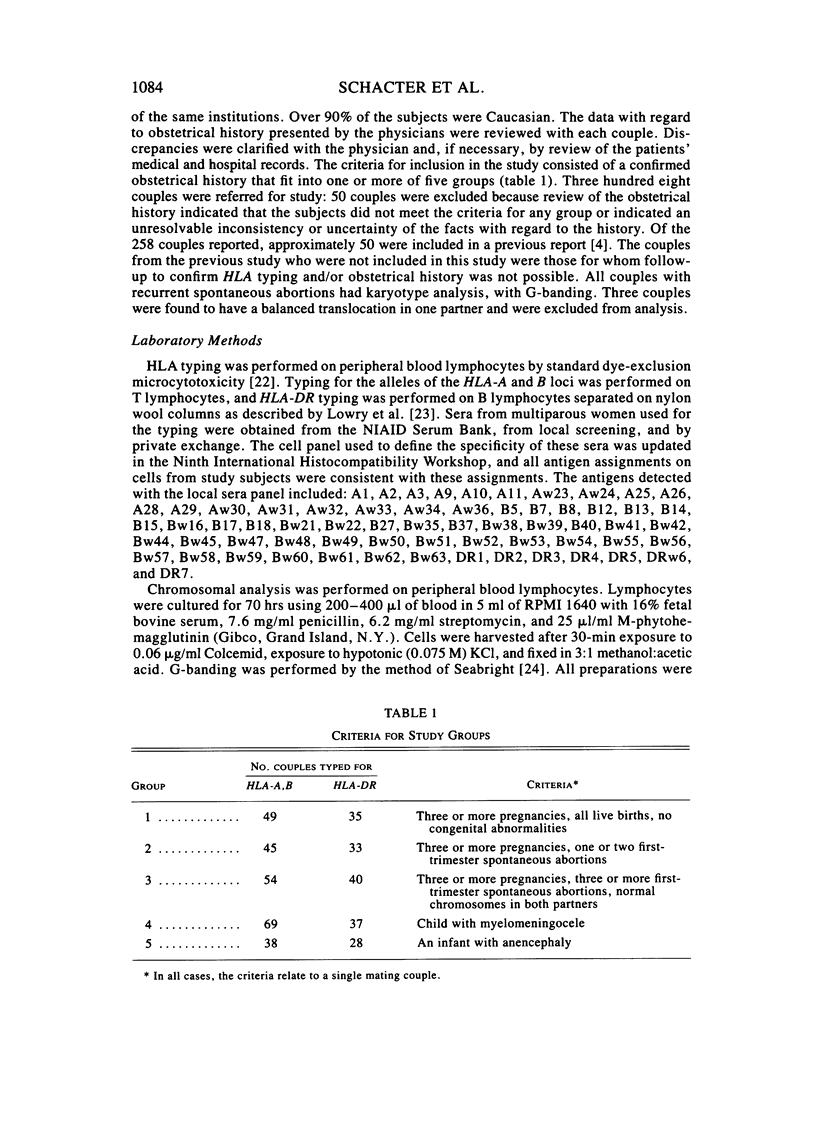
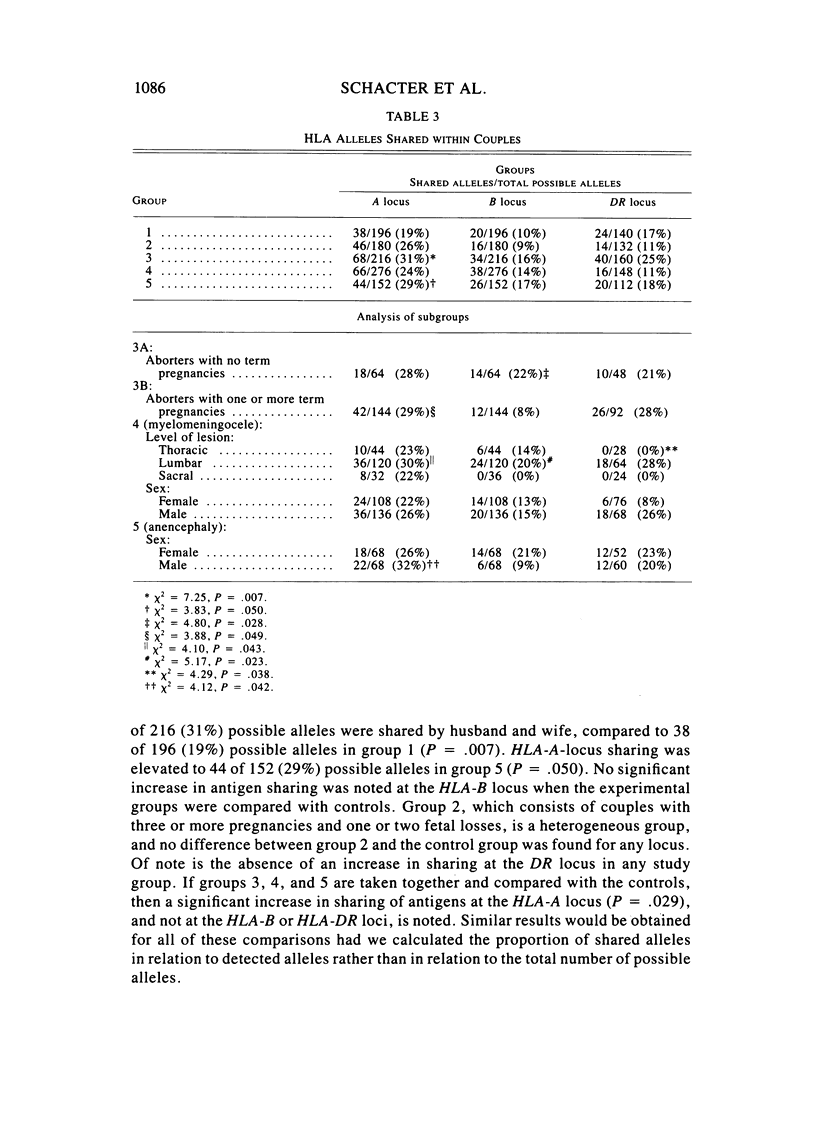
To test the hypothesis that a locus in or near the human major histocompatibility complex (HLA) contributes to both involuntary fetal loss and neural tube defects (NTD), we evaluated sharing of antigens of the HLA-A, HLA-B, or HLA-DR loci of couples who had three or more first-trimester spontaneous abortions or who had a child with an NTD (myelomeningocele or anencephaly). HLA-A antigen sharing was increased in couples with three or more spontaneous abortions and in couples who had an anencephalic fetus, when compared with couples who had three or more pregnancies and no fetal loss. Increased sharing of antigens at the HLA-A and B loci was not seen in the entire group of couples with children with myelomeningocele, but was found in the subgroup of 36 couples whose child had a lumbar myelomeningocele. An increase in HLA-DR sharing was not seen in any group or subgroup when compared with the control couples. Among the aborting couples, increased sharing was not restricted to the couples who had no term pregnancies, but was also found in the couples whose fetal losses occurred after one or more normal term pregnancies. These results are consistent with the hypothesis that a locus on the HLA-A side of the HLA-DR locus contributes to some fetal loss and susceptibility to NTD. This model is proposed as an alternative to the hypothesis that the maternal immune response to paternal major histocompatibility complex (MHC) antigens is the basis for increased HLA sharing in couples with fetal wastage.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert E. D., Mickey M. R., Ting A., Terasaki P. I. Deduction of 2140 HL-A haplotypes and segregation analysis in 535 families. Transplant Proc. 1973 Mar;5(1):215–221. [PubMed] [Google Scholar]
- Awdeh Z. L., Raum D., Yunis E. J., Alper C. A. Extended HLA/complement allele haplotypes: evidence for T/t-like complex in man. Proc Natl Acad Sci U S A. 1983 Jan;80(1):259–263. doi: 10.1073/pnas.80.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer A. E., Quebbeman J. F., Ayers J. W., Haines R. F. Major histocompatibility complex antigens, maternal and paternal immune responses, and chronic habitual abortions in humans. Am J Obstet Gynecol. 1981 Dec 15;141(8):987–999. doi: 10.1016/s0002-9378(16)32690-4. [DOI] [PubMed] [Google Scholar]
- Bobrow M., Bodmer J. G., Bodmer W. F., McDevitt H. O., Lorber J., Swift P. The search for a human equivalent of the mouse T-locus - negative results from a study of HL-A types in spina bifida. Tissue Antigens. 1975 May;5(4):234–237. doi: 10.1111/j.1399-0039.1975.tb01469.x. [DOI] [PubMed] [Google Scholar]
- De Bruyere M., Kulakowski S., Malchaire J., Delire M., Sokal G. HLA gene and haplotype frequencies in spina bifida. Population and family studies. Tissue Antigens. 1977 Nov;10(5):399–402. doi: 10.1111/j.1399-0039.1977.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Temple A. Distribution of beta2 microglobulin and HLA in chorionic villi of human placentae. Nature. 1976 Aug 26;262(5571):799–802. doi: 10.1038/262799a0. [DOI] [PubMed] [Google Scholar]
- Gerencer M., Kastelan A., Drazancić A., Kerhin-Brkljacić V., Madjarić M. The HLA antigens in women with recurrent abnormal pregnancies of unknown etiology. Tissue Antigens. 1978 Sep;12(3):223–227. doi: 10.1111/j.1399-0039.1978.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd Immunogenetics of spontaneous abortions in humans. Transplantation. 1983 Jan;35(1):1–6. [PubMed] [Google Scholar]
- Goodfellow P. N., Barnstable C. J., Bodmer W. F., Snary D., Crumpton M. J. Expression of HLA system antigens on placenta. Transplantation. 1976 Dec;22(6):595–603. doi: 10.1097/00007890-197612000-00009. [DOI] [PubMed] [Google Scholar]
- Hammerberg C., Klein J. Linkage disequilibrium between H-2 and t complexes in chromosome 17 of the mouse. Nature. 1975 Nov 27;258(5533):296–299. doi: 10.1038/258296a0. [DOI] [PubMed] [Google Scholar]
- Holmes L. B., Driscoll S. G., Atkins L. Etiologic heterogeneity of neural-tube defects. N Engl J Med. 1976 Feb 12;294(7):365–369. doi: 10.1056/NEJM197602122940704. [DOI] [PubMed] [Google Scholar]
- James W. H. The sex ratio in anencephaly. J Med Genet. 1979 Apr;16(2):129–133. doi: 10.1136/jmg.16.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Hammerberg C. The control of differentiation by the T complex. Immunol Rev. 1977 Jan;33:70–104. doi: 10.1111/j.1600-065x.1977.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Komlos L., Zamir R., Joshua H., Halbrecht I. Common HLA antigens in couples with repeated abortions. Clin Immunol Immunopathol. 1977 May;7(3):330–335. doi: 10.1016/0090-1229(77)90066-6. [DOI] [PubMed] [Google Scholar]
- Lauritsen J. G., Jøorgensen J., Kissmeyer-Nielsen F. Significance of HLA and blood-group incompatibility in spontaneous abortion. Clin Genet. 1976 Jun;9(6):575–582. doi: 10.1111/j.1399-0004.1976.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Leck I. Causation of neural tube defects: clues from epidemiology. Br Med Bull. 1974 May;30(2):158–163. doi: 10.1093/oxfordjournals.bmb.a071187. [DOI] [PubMed] [Google Scholar]
- Lowry R., Goguen J., Carpenter C. B., Strom T. B., Garovoy M. R. Improved B cell typing for HLA-DR using nylon wool column enriched B lymphocyte preparations. Tissue Antigens. 1979 Oct;14(4):325–330. doi: 10.1111/j.1399-0039.1979.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Mayr W. Die Genetik des HL-A Systems. Populations und Familienuntersuchungen, unter besonderer Berücksichtigung der Paternitätsserologie. Humangenetik. 1971;12(3):195–243. doi: 10.1007/BF00702775. [DOI] [PubMed] [Google Scholar]
- Montgomery B., Lala P. K. Ontogeny of the MHC antigens on human trophoblast cells during the first trimester of pregnancy. J Immunol. 1983 Nov;131(5):2348–2355. [PubMed] [Google Scholar]
- Ober C. L., Martin A. O., Simpson J. L., Hauck W. W., Amos D. B., Kostyu D. D., Fotino M., Allen F. H., Jr Shared HLA antigens and reproductive performance among Hutterites. Am J Hum Genet. 1983 Sep;35(5):994–1004. [PMC free article] [PubMed] [Google Scholar]
- Palm J. Proceedings: Maternal-fetal histoincompatibility in rats: an escape from adversity. Cancer Res. 1974 Aug;34(8):2061–2065. [PubMed] [Google Scholar]
- Rocklin R. E., Kitzmiller J. L., Carpenter C. B., Garovoy M. R., David J. R. Maternal-fetal relation. Absence of an immunologic blocking factor from the serum of women with chronic abortions. N Engl J Med. 1976 Nov 25;295(22):1209–1213. doi: 10.1056/NEJM197611252952201. [DOI] [PubMed] [Google Scholar]
- Schacter B., Muir A., Gyves M., Tasin M. HLA-A,B compatibility in parents of offspring with neural-tube defects or couples experiencing involuntary fetal wastage. Lancet. 1979 Apr 14;1(8120):796–799. doi: 10.1016/s0140-6736(79)91317-5. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Smithells R. W., Sheppard S., Schorah C. J., Seller M. J., Nevin N. C., Harris R., Read A. P., Fielding D. W. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet. 1980 Feb 16;1(8164):339–340. doi: 10.1016/s0140-6736(80)90886-7. [DOI] [PubMed] [Google Scholar]
- Sunderland C. A., Redman C. W., Stirrat G. M. HLA A, B, C antigens are expressed on nonvillous trophoblast of the early human placenta. J Immunol. 1981 Dec;127(6):2614–2615. [PubMed] [Google Scholar]
- Taylor C., Faulk W. P. Prevention of recurrent abortion with leucocyte transfusions. Lancet. 1981 Jul 11;2(8237):68–70. doi: 10.1016/s0140-6736(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Vannier J. P., Cavelier B., Martin J. P., Lefort J., Rivat L., Feingold J. HLA, Pi, Gm and Km phenotypes in a spina bifida population with myelo-meningocele. Tissue Antigens. 1980 May;15(5):501–504. doi: 10.1111/j.1399-0039.1980.tb00214.x. [DOI] [PubMed] [Google Scholar]


