Abstract
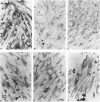
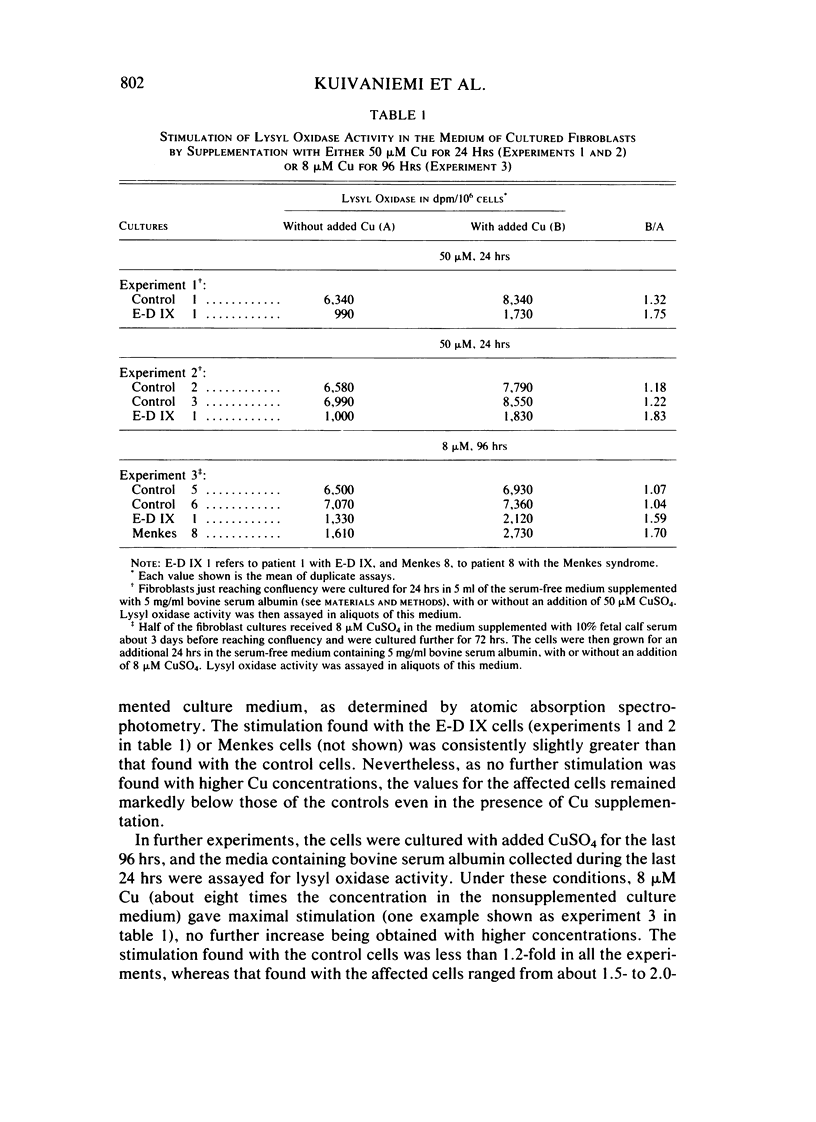
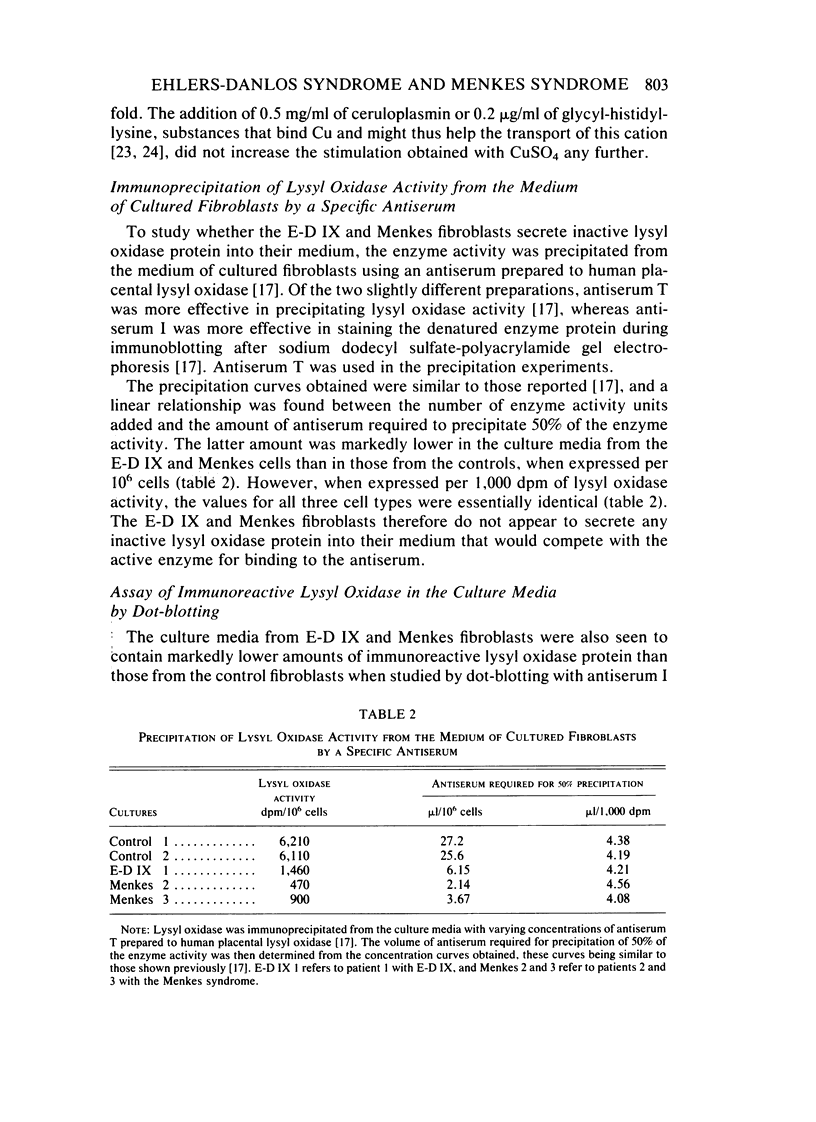
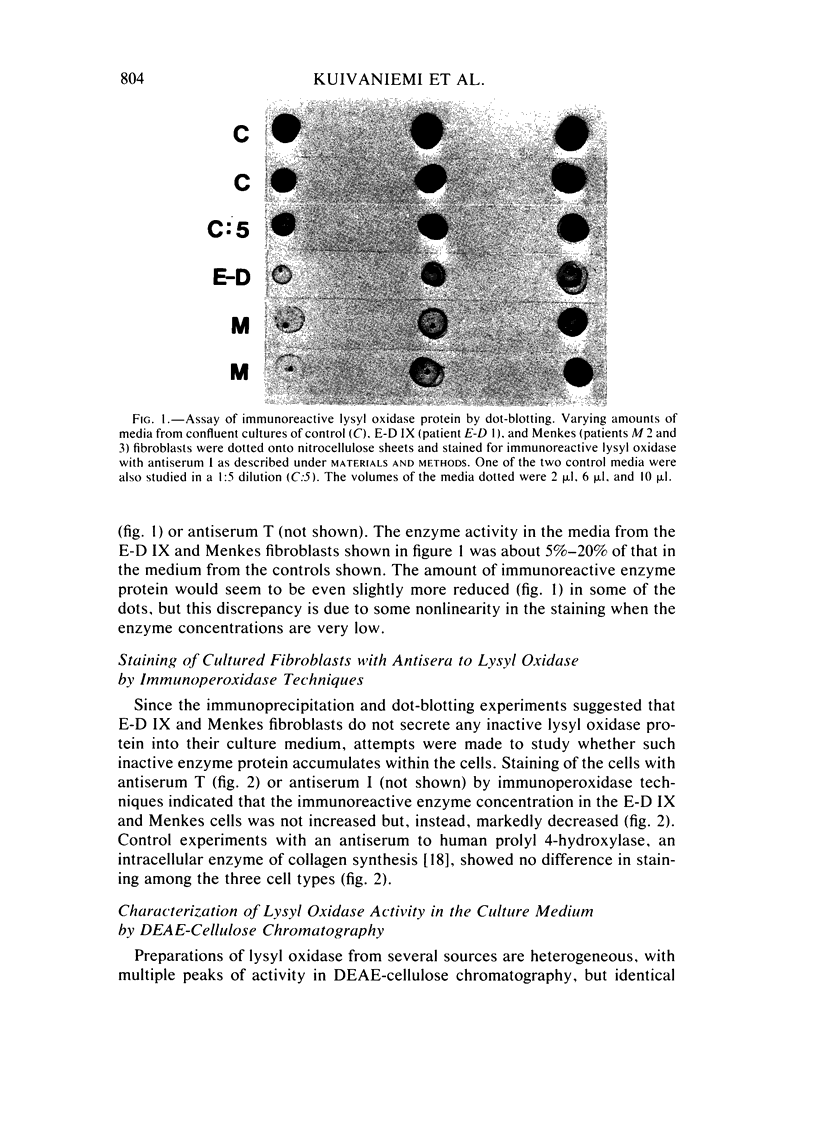
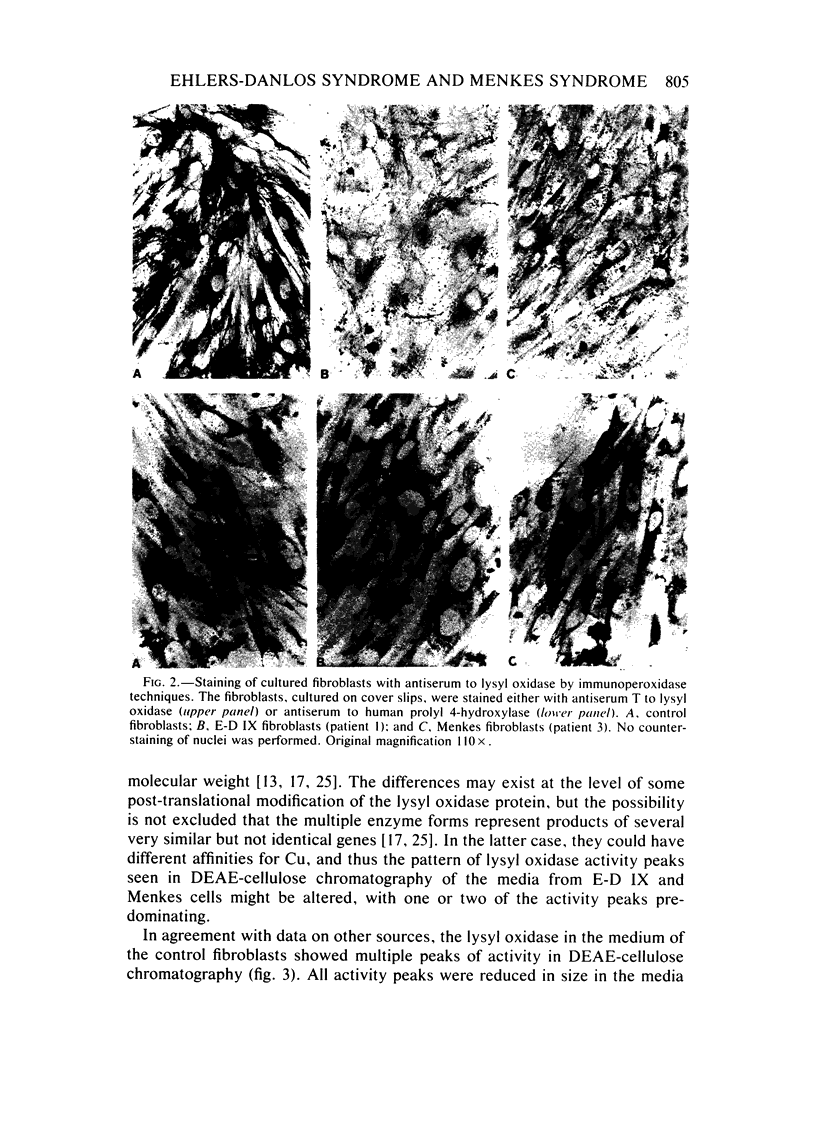
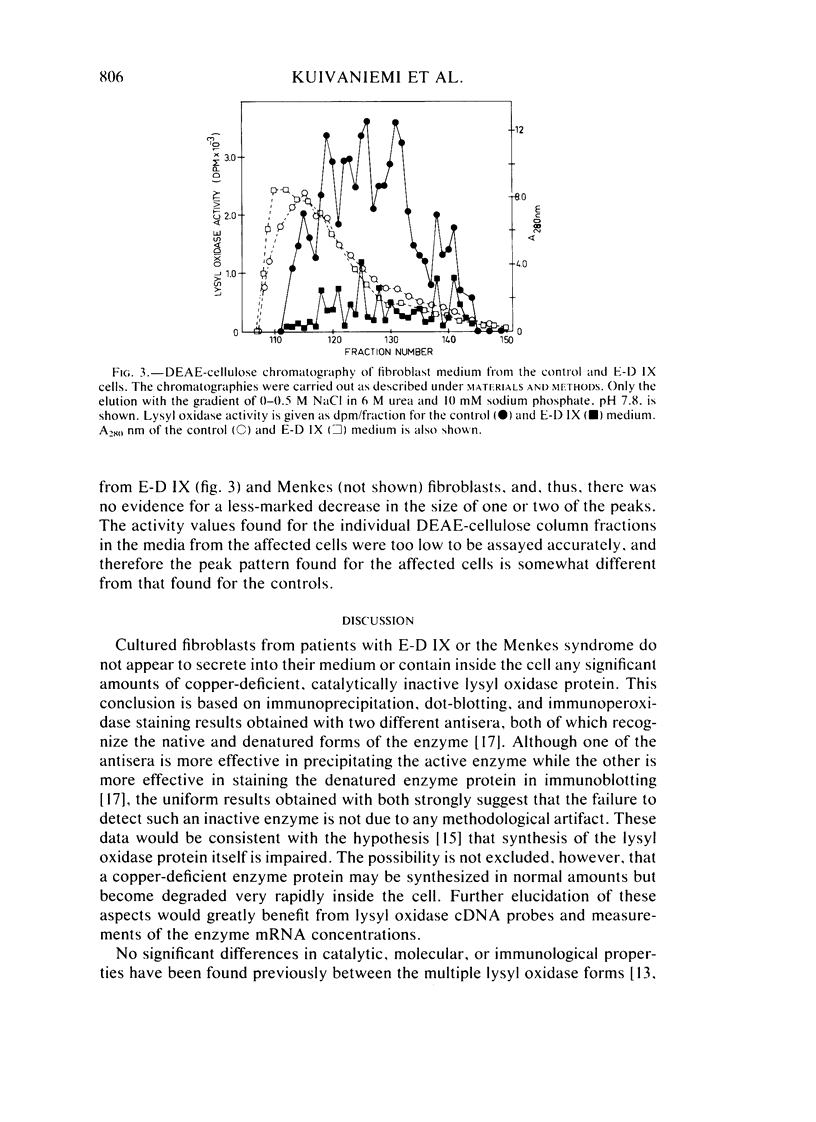
Type IX of the Ehlers-Danlos syndrome (E-D IX) and the Menkes syndrome are X-linked recessively inherited disorders characterized by abnormalities in copper metabolism. These abnormalities are associated with a severe reduction in the activity of lysyl oxidase, the extracellular copper enzyme that initiates crosslinking of collagens and elastin. No increase in this deficient enzyme activity was obtained when culture media from fibroblasts of patients with E-D IX or the Menkes syndrome were incubated with copper under various conditions in vitro. A distinct, although small, increase in lysyl oxidase activity was obtained, however, when copper-supplemented media were used during culturing of the fibroblasts, although even under these conditions, the enzyme activity in the media from the affected cells remained markedly below that of the controls. Immunoprecipitation, dot-blotting, and immunoperoxidase staining experiments with antisera to human lysyl oxidase indicated that fibroblasts from patients with E-D IX or the Menkes syndrome do not secrete into their medium, or contain inside the cell, any significant amounts of a copper-deficient, catalytically inactive lysyl oxidase protein. These findings appear to be consistent with the hypothesis that synthesis of the lysyl oxidase protein itself is impaired. The possibility is not excluded, however, that a copper-deficient enzyme protein may be synthesized in normal amounts but become degraded very rapidly inside the cell. The failure to obtain any large increase in the deficient lysyl oxidase activity upon various forms of copper administration suggests that it may not be possible to obtain any significant improvement in the connective tissue manifestations of these disorders by copper therapy.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byers P. H., Siegel R. C., Holbrook K. A., Narayanan A. S., Bornstein P., Hall J. G. X-linked cutis laxa: defective cross-link formation in collagen due to decreased lysyl oxidase activity. N Engl J Med. 1980 Jul 10;303(2):61–65. doi: 10.1056/NEJM198007103030201. [DOI] [PubMed] [Google Scholar]
- Harris E. D., DiSilvestro R. A. Correlation of lysyl oxidase activation with the p-phenylenediamine oxidase activity (ceruloplasmin) in serum. Proc Soc Exp Biol Med. 1981 Apr;166(4):528–531. doi: 10.3181/00379727-166-41102. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Posttranslational enzymes in the biosynthesis of collagen: intracellular enzymes. Methods Enzymol. 1982;82(Pt A):245–304. doi: 10.1016/0076-6879(82)82067-3. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Peltonen L., Palotie A., Kaitila I., Kivirikko K. I. Abnormal copper metabolism and deficient lysyl oxidase activity in a heritable connective tissue disorder. J Clin Invest. 1982 Mar;69(3):730–733. doi: 10.1172/JCI110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuivaniemi H., Savolainen E. R., Kivirikko K. I. Human placental lysyl oxidase. Purification, partial characterization, and preparation of two specific antisera to the enzyme. J Biol Chem. 1984 Jun 10;259(11):6996–7002. [PubMed] [Google Scholar]
- Peltonen L., Kuivaniemi H., Palotie A., Horn N., Kaitila I., Kivirikko K. I. Alterations in copper and collagen metabolism in the Menkes syndrome and a new subtype of the Ehlers-Danlos syndrome. Biochemistry. 1983 Dec 20;22(26):6156–6163. doi: 10.1021/bi00295a018. [DOI] [PubMed] [Google Scholar]
- Pickart L., Freedman J. H., Loker W. J., Peisach J., Perkins C. M., Stenkamp R. E., Weinstein B. Growth-modulating plasma tripeptide may function by facilitating copper uptake into cells. Nature. 1980 Dec 25;288(5792):715–717. doi: 10.1038/288715a0. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., McGoodwin E. B., Martin G. R., Grahn D. Decreased lysyl oxidase activity in the aneurysm-prone, mottled mouse. J Biol Chem. 1977 Feb 10;252(3):939–942. [PubMed] [Google Scholar]
- Royce P. M., Camakaris J., Mann J. R., Danks D. M. Copper metabolism in mottled mouse mutants. The effect of copper therapy on lysyl oxidase activity in brindled (Mobr) mice. Biochem J. 1982 Feb 15;202(2):369–371. doi: 10.1042/bj2020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. C. Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4826–4830. doi: 10.1073/pnas.71.12.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcher B., Madaras J. A., Fisk D., Perry E. F., Hill C. H. Abnormal cellular copper metabolism in the blotchy mouse. J Nutr. 1978 Aug;108(8):1229–1233. doi: 10.1093/jn/108.8.1229. [DOI] [PubMed] [Google Scholar]
- Sternberg J., Jeppesen P. Dot-blotting--a novel screening assay for antibodies in hybridoma cultures. J Immunol Methods. 1983 Nov 11;64(1-2):39–43. doi: 10.1016/0022-1759(83)90382-4. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A. The unlabeled antibody method. Hormone receptor, Golgi-like and dual color immunocytochemistry. J Histochem Cytochem. 1979 Dec;27(12):1658–1659. doi: 10.1177/27.12.230258. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Kagan H. M. Evidence for structural similarities in the multiple forms of aortic and cartilage lysyl oxidase and a catalytically quiescent aortic protein. J Biol Chem. 1982 Nov 25;257(22):13520–13526. [PubMed] [Google Scholar]
- Zeller R., Nyffenegger T., De Robertis E. M. Nucleocytoplasmic distribution of snRNPs and stockpiled snRNA-binding proteins during oogenesis and early development in Xenopus laevis. Cell. 1983 Feb;32(2):425–434. doi: 10.1016/0092-8674(83)90462-2. [DOI] [PubMed] [Google Scholar]