Abstract
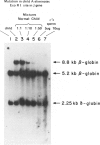
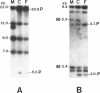
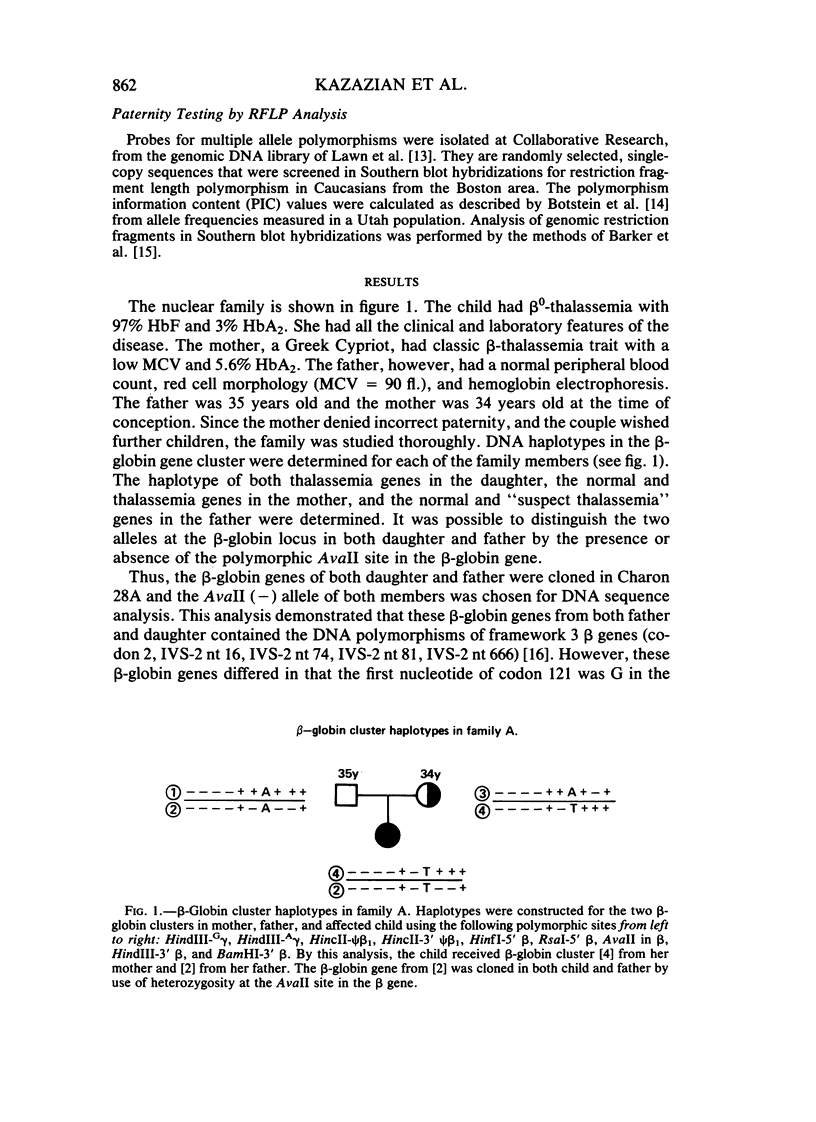
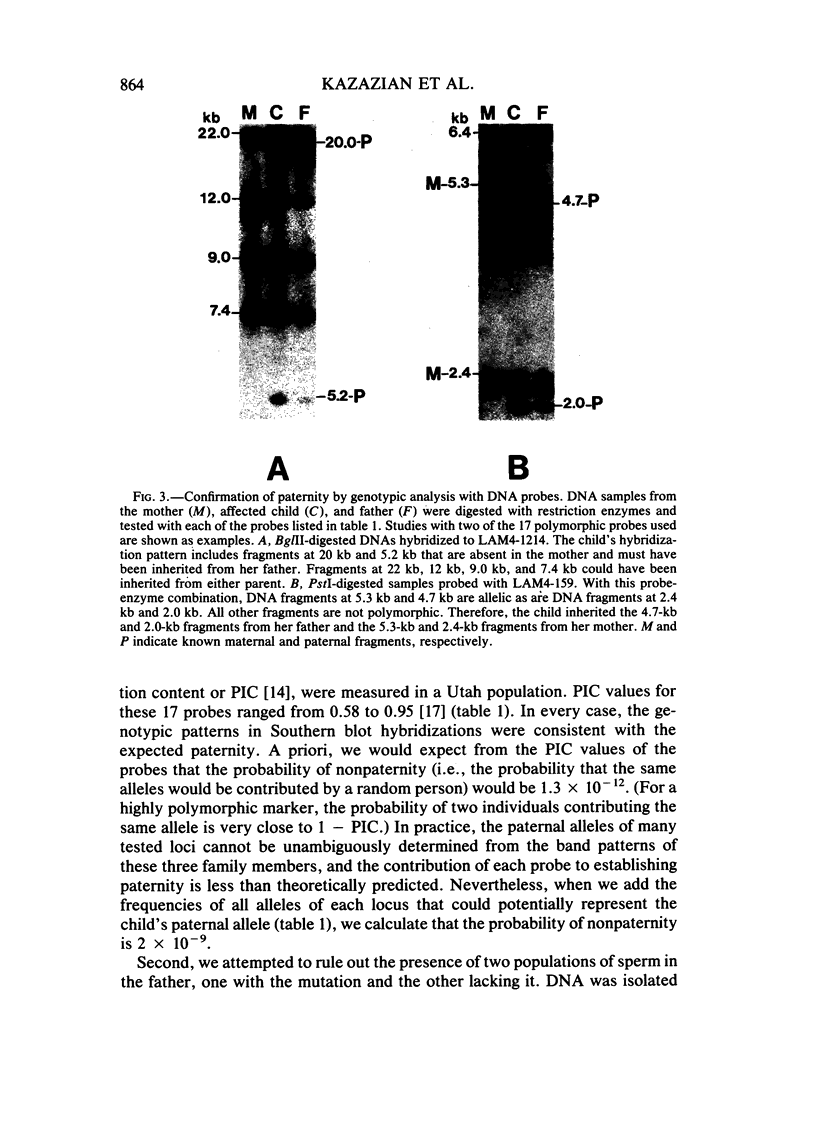
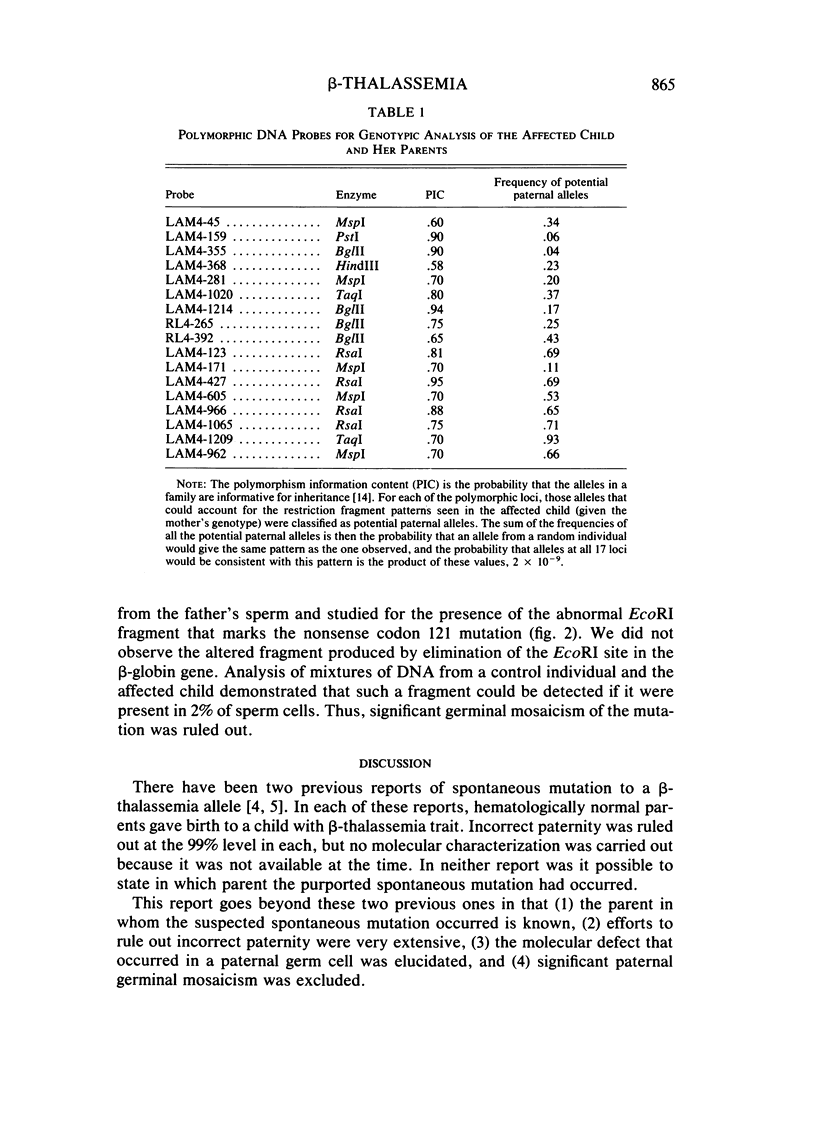
We have studied a nuclear family containing a single child with severe beta-thalassemia intermedia, a Greek-Cypriot mother with hematological findings of beta-thalassemia trait, and a Polish father who is hematologically normal. Since both the child and her father were heterozygous for a DNA polymorphism within the beta-globin gene, it was possible to clone and sequence the beta-globin gene identical by descent from both the child and her father. A nonsense mutation in codon 121 (GAA----TAA) was found in the beta-globin gene of the child, while the same gene from her father lacked this mutation and was normal. This mutation has not been previously observed among over 200 beta-thalassemia genes characterized in Caucasians. Since the mutation eliminates an EcoRI site in the beta-globin gene, we could show that the mutation is not present in genomic DNA of the father. To rule out germinal mosaicism, sperm DNA of the father was also digested with EcoRI, and the mutant EcoRI fragment was not observed under conditions that would detect the mutation if it were present in at least 2% of sperm cells. Routine HLA and blood group testing supported stated paternity. In addition, studies with 17 DNA probes that detect multiple allele polymorphisms increased the probability of stated paternity to at least 10(8):1. These data provide evidence that the G----T change in codon 121 of the beta-globin gene in the child is the result of a spontaneous mutation that occurred during spermatogenesis in a paternal germ cell.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAGLIONI C. Abnormal human haemoglobins. VII. Chemical studies on haemoglobin D. Biochim Biophys Acta. 1962 May 21;59:437–449. doi: 10.1016/0006-3002(62)90194-4. [DOI] [PubMed] [Google Scholar]
- BAGLIONI C., LEHMANN H. Chemical heterogeneity of haemoglobin O. Nature. 1962 Oct 20;196:229–232. doi: 10.1038/196229a0. [DOI] [PubMed] [Google Scholar]
- Barker D., Holm T., White R. A locus on chromosome 11p with multiple restriction site polymorphisms. Am J Hum Genet. 1984 Nov;36(6):1159–1171. [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Blechl A. E., Denniston-Thompson K., Faber H. E., Richards J. E., Slightom J. L., Tucker P. W., Smithies O. Cloning human fetal gamma globin and mouse alpha-type globin DNA: preparation and screening of shotgun collections. Science. 1978 Dec 22;202(4374):1279–1284. doi: 10.1126/science.725603. [DOI] [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti A., Buetow K. H., Antonarakis S. E., Waber P. G., Boehm C. D., Kazazian H. H. Nonuniform recombination within the human beta-globin gene cluster. Am J Hum Genet. 1984 Nov;36(6):1239–1258. [PMC free article] [PubMed] [Google Scholar]
- Davie A. M., Emery A. E. Estimation of proportion of new mutants among cases of Duchenne muscular dystrophy. J Med Genet. 1978 Oct;15(5):339–345. doi: 10.1136/jmg.15.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Felsenstein J., Gartler S. M., Migeon B. R., Dancis J., Seegmiller J. E., Bakay F., Nyhan W. L. The occurrence of new mutants in the X-linked recessive Lesch-Nyhan disease. Am J Hum Genet. 1976 Mar;28(2):123–137. [PMC free article] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Tuddenham E. G., Shuman M. A., Goralka T. M., Chen E. Y., Lawn R. M. Detection and sequence of mutations in the factor VIII gene of haemophiliacs. 1985 May 30-Jun 5Nature. 315(6018):427–430. doi: 10.1038/315427a0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Antonarakis S. E., Sexton J. P., Boehm C. D., Goff S. C., Waber P. G. Molecular characterization of seven beta-thalassemia mutations in Asian Indians. EMBO J. 1984 Mar;3(3):593–596. doi: 10.1002/j.1460-2075.1984.tb01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Orkin S. H., Markham A. F., Chapman C. R., Youssoufian H., Waber P. G. Quantification of the close association between DNA haplotypes and specific beta-thalassaemia mutations in Mediterraneans. Nature. 1984 Jul 12;310(5973):152–154. doi: 10.1038/310152a0. [DOI] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nronha P. A., Honig G. R. beta-Thalassemia arising as a new mutation in an American child. Am J Hematol. 1978;4(2):187–192. doi: 10.1002/ajh.2830040211. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr, Antonarakis S. E., Goff S. C., Boehm C. D., Sexton J. P., Waber P. G., Giardina P. J. Linkage of beta-thalassaemia mutations and beta-globin gene polymorphisms with DNA polymorphisms in human beta-globin gene cluster. Nature. 1982 Apr 15;296(5858):627–631. doi: 10.1038/296627a0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Kazazian H. H., Jr The mutation and polymorphism of the human beta-globin gene and its surrounding DNA. Annu Rev Genet. 1984;18:131–171. doi: 10.1146/annurev.ge.18.120184.001023. [DOI] [PubMed] [Google Scholar]
- PENROSE L. S. Parental age in acondroplasia and mongolism. Am J Hum Genet. 1957 Sep;9(3):167–169. [PMC free article] [PubMed] [Google Scholar]
- Tönz O., Glatthaar B. E., Winterhalter K. H., Ritter H. New mutation in a Swiss girl leading to clinical and biochemical beta-thalassemia minor. Humangenetik. 1973 Dec 20;20(4):321–327. doi: 10.1007/BF00273335. [DOI] [PubMed] [Google Scholar]
- Yang T. P., Patel P. I., Chinault A. C., Stout J. T., Jackson L. G., Hildebrand B. M., Caskey C. T. Molecular evidence for new mutation at the hprt locus in Lesch-Nyhan patients. Nature. 1984 Aug 2;310(5976):412–414. doi: 10.1038/310412a0. [DOI] [PubMed] [Google Scholar]