Abstract
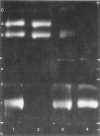
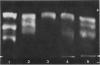
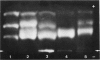
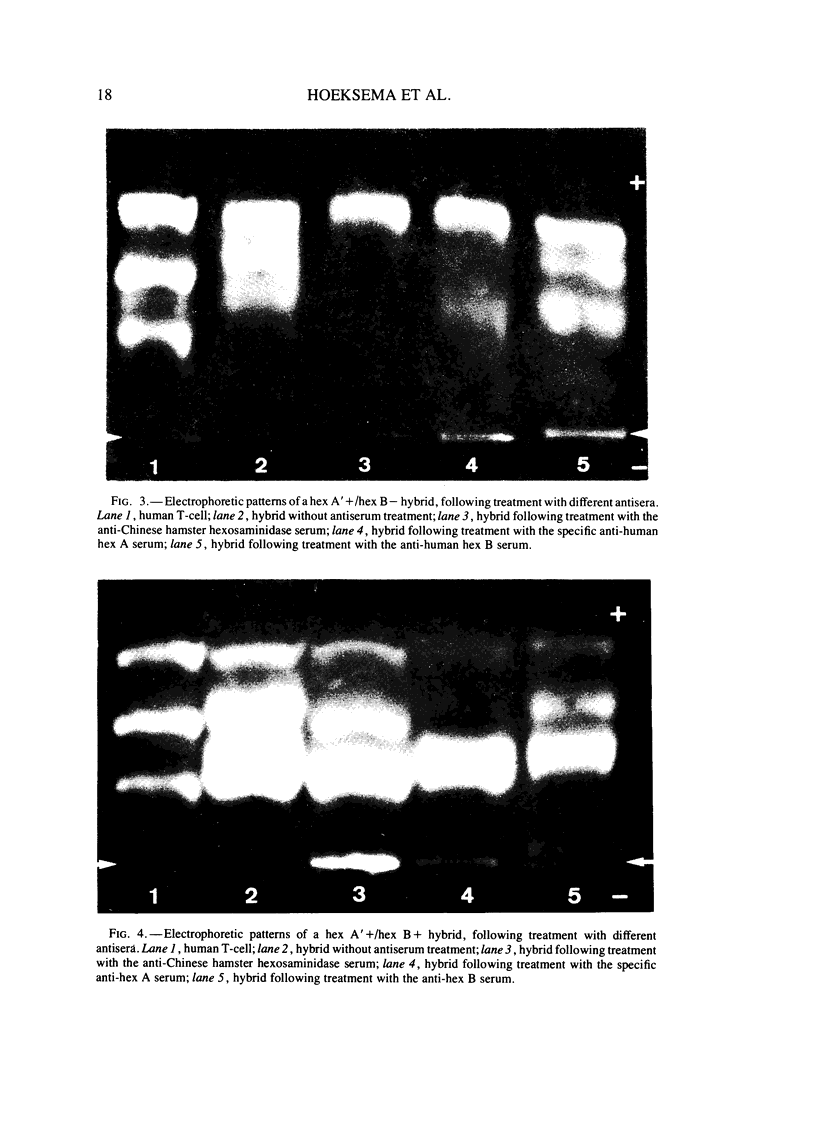
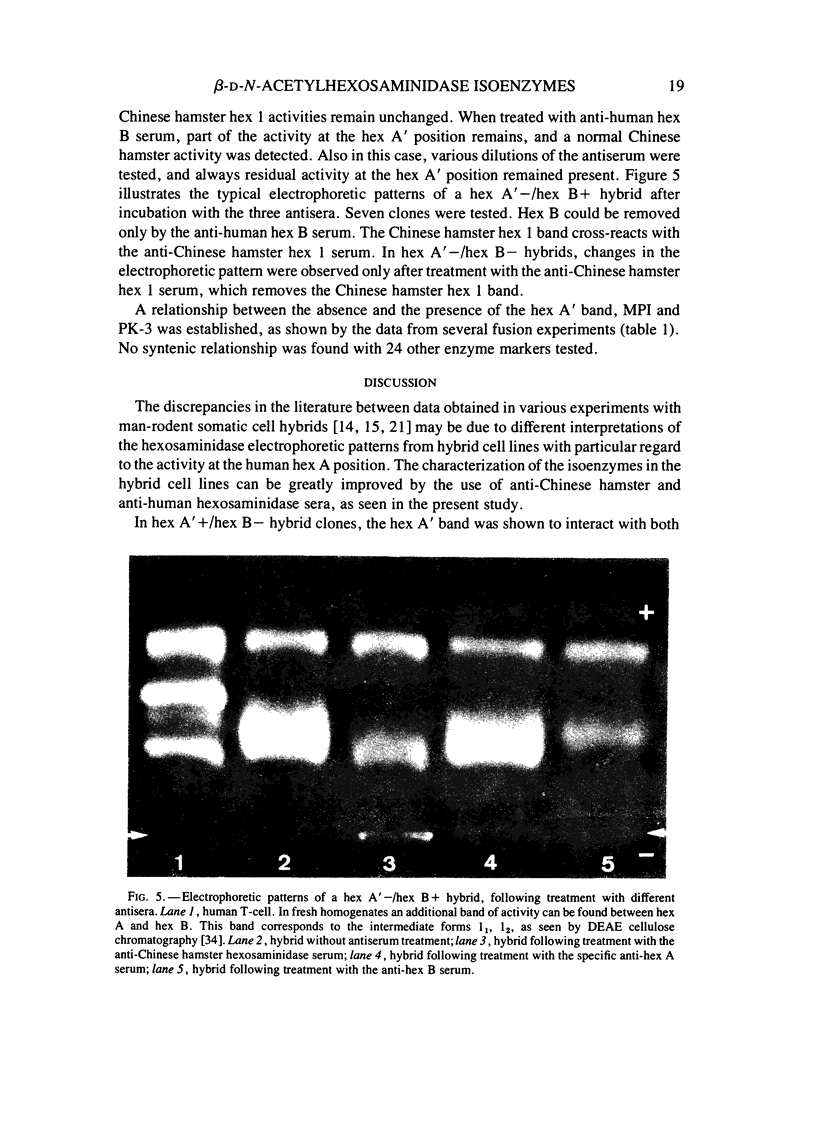
A series of man-Chinese hamster hybrids were investigated with the use of an anti-Chinese hamster hexosaminidase serum, a specific anti-human hex A serum and an anti-human hex B serum. The expression of human hex A was found to be dependent on the presence of hex B. A heteropolymeric molecule is formed independently of hex B, which consists of Chinese hamster and specific hex A moieties. It has an electrophoretic mobility nearly identical to hex A. A relationship between the absence and presence of the heteropolymeric molecule, mannosephosphate isomerase (MPI), and pyruvate kinase (PK-3), assigned to chromosome 15, was established. With respect to the two locus subunit model, the gene coding for the alpha subunit, specific for hex A, has been localized on chromosome 15.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew W. R., Rattazzi M. C. Immunochemical characterization of human beta-D-N-acetyl hexosaminidase from normal individuals and patients with Tay-Sachs disease. I. Antigenic differences between hexosaminidase A and hexosaminidase B. Int Arch Allergy Appl Immunol. 1974;46(4):512–524. doi: 10.1159/000231154. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Subunit structure of human hexosaminidase verified: interconvertibility of hexosaminidase isozymes. Nature. 1975 Nov 20;258(5532):262–264. doi: 10.1038/258262a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., Srivastava S. K. Studies in Tay-Sachs and Sandhoff's diseases. Immunologic and structural properties of hexosaminidase A and hexosaminidase B. Isr J Med Sci. 1973 Sep-Oct;9(9):1335–1337. [PubMed] [Google Scholar]
- Carroll M., Robinson D. A low-molecular-weight protein cross-reacting with human liver N-acetyl-beta-D-glucosaminidase. Biochem J. 1974 Feb;137(2):217–221. doi: 10.1042/bj1370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjaard H., Hoogeveen A., de Wit-Verbeek H. A., Reuser A. J., Keijzer W., Westerveld A., Bootsma D. Tay-Sachs and Sandhoff's disease: intergenic complementation after somatic cell hybridization. Exp Cell Res. 1974 Aug;87(2):444–448. doi: 10.1016/0014-4827(74)90515-1. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Kucherlapati R., Creagan R. P., Murnane M. J., Darlington G. J., Ruddle F. H. Tay-Sachs' and Sandhoff's diseases: the assignment of genes for hexosaminidase A and B to individual human chromosomes. Proc Natl Acad Sci U S A. 1975 Jan;72(1):263–267. doi: 10.1073/pnas.72.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooghwinkel G. J., Veltkamp W. A., Overdijk B., Lisman J. J. Electrophoretic separation of -N-acetylhexosaminidases of human and bovine brain and liver and of Tay-Sachs brain tissue. Hoppe Seylers Z Physiol Chem. 1972 May;353(5):839–841. [PubMed] [Google Scholar]
- Ikonne J. U., Rattazzi M. C., Desnick R. J. Characterization of Hex S, the major residual beta hexosaminidase activity in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet. 1975 Sep;27(5):639–650. [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Proceedings: Synteny relationships of beta-D-N-acetylhexosaminidase A and B in somatic cell hybrids. Cytogenet Cell Genet. 1974;13(1):111–116. doi: 10.1159/000130250. [DOI] [PubMed] [Google Scholar]
- Lotan R., Gussin A. E., Lis H., Sharon N. Purification of wheat germ agglutinin by affinity chromatography on a sepharose-bound N-acetylglucosamine derivative. Biochem Biophys Res Commun. 1973 May 15;52(2):656–662. doi: 10.1016/0006-291x(73)90763-8. [DOI] [PubMed] [Google Scholar]
- Meera Khan P. Enzyme electrophoresis on cellulose acetate gel: zymogram patterns in mgh-mouse and man--Chinese hamster somatic cell hybrids. Arch Biochem Biophys. 1971 Aug;145(2):470–483. doi: 10.1016/s0003-9861(71)80007-3. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Okada S., Ho M. W., Fillerup D. L., Veath M. L., Adams K. Ganglioside storage diseases. Fed Proc. 1971 May-Jun;30(3):956–969. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Price R. G., Dance N. The demonstration of multiple heat stable forms of N-acetyl- -glucosaminidase in normal human serum. Biochim Biophys Acta. 1972 Jun 22;271(1):145–153. doi: 10.1016/0005-2795(72)90142-0. [DOI] [PubMed] [Google Scholar]
- Robinson D., Carroll M. Tay-Sachs disease: interrelation of hexosaminidases A and B. Lancet. 1972 Feb 5;1(7745):322–323. doi: 10.1016/s0140-6736(72)90332-7. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropers H. H., Schwantes U. On the molecular basis of Sandhoff's disease. Humangenetik. 1973;20(2):167–170. doi: 10.1007/BF00284854. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Thomas G. H., Taylor H. A., Miller C. S., Axelman J., Migeon B. R. Genetic complementation after fusion of Tay-Sachs and Sandhoff cells. Nature. 1974 Aug 16;250(467):580–582. doi: 10.1038/250580a0. [DOI] [PubMed] [Google Scholar]
- VAN DER VEEN J., BOTS L., MES A. Establishment of two human cell strains from kidney and reticulosarcoma in lung. Arch Gesamte Virusforsch. 1958;8(2):230–238. doi: 10.1007/BF01241484. [DOI] [PubMed] [Google Scholar]
- Van Cong N., Weil D., Rebourcet R., Frézal J., Richard-Mollard A. M. A study of hexosaminadases in interspecific hybrids and in GM2 gangliosidosis with a discussion on their genetic control. Ann Hum Genet. 1975 Jul;39(1):111–123. doi: 10.1111/j.1469-1809.1975.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Westerveld A., Visser R. P., Meera Khan P., Bootsma D. Loss of human genetic markers in man--Chinese hamster somatic cell hybrids. Nat New Biol. 1971 Nov 3;234(44):20–24. doi: 10.1038/newbio234020a0. [DOI] [PubMed] [Google Scholar]
- Westerveld A., van Someren H., van Henegouwen H. M., Oosterbaan R. A. Synteny relationship between the human loci for hexosaminidase-a, mannose phosphate isomerase, and pyruvate kinase-3 studied in man-Chinese hamster somatic cell hybrids. Birth Defects Orig Artic Ser. 1975;11(3):285–287. [PubMed] [Google Scholar]
- van Heyningen V., Bobrow M., Bodmer W. F., Gardiner S. E., Povey S., Hopkinson D. A. Chromosome assignment of some human enzyme loci: mitochondrial malate dehydrogenase to 7, mannosephosphate isomerase and pyruvate kinase to 15 and probably, esterase D to 13. Ann Hum Genet. 1975 Jan;38(3):295–303. doi: 10.1111/j.1469-1809.1975.tb00613.x. [DOI] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegou Independent loss of human hexosaminidases A and B in man-chinese hamster somatic cell hybrids. Humangenetik. 1973 Apr 16;18(2):171–174. doi: 10.1007/BF00291485. [DOI] [PubMed] [Google Scholar]
- van Someren H., Beijersbergen van Henegouwen H., Los W., Wurzer-Figurelli E., Doppert B., Vervloet M., Meera Khan P. Enzyme electrophoresis on cellulose acetate gel. II. Zymogram patterns in man-Chinese hamster somatic cell hybrids. Humangenetik. 1974;25(3):189–201. doi: 10.1007/BF00281426. [DOI] [PubMed] [Google Scholar]