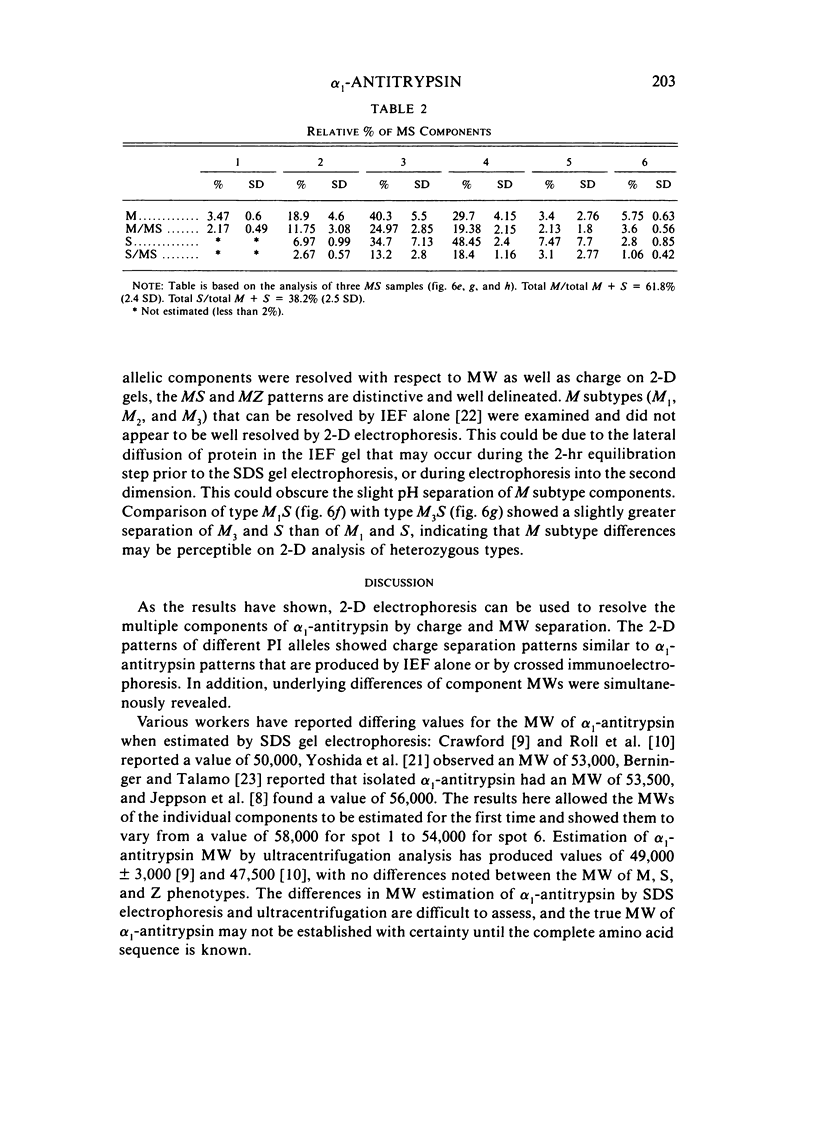
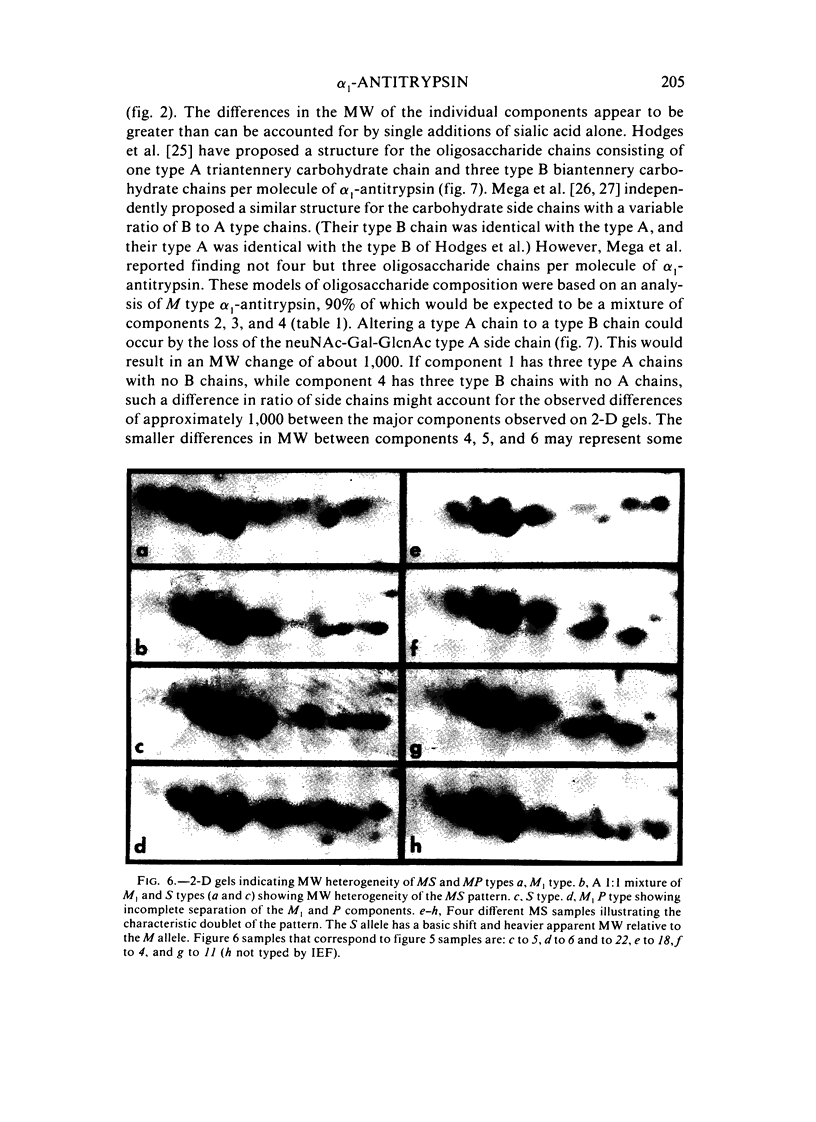
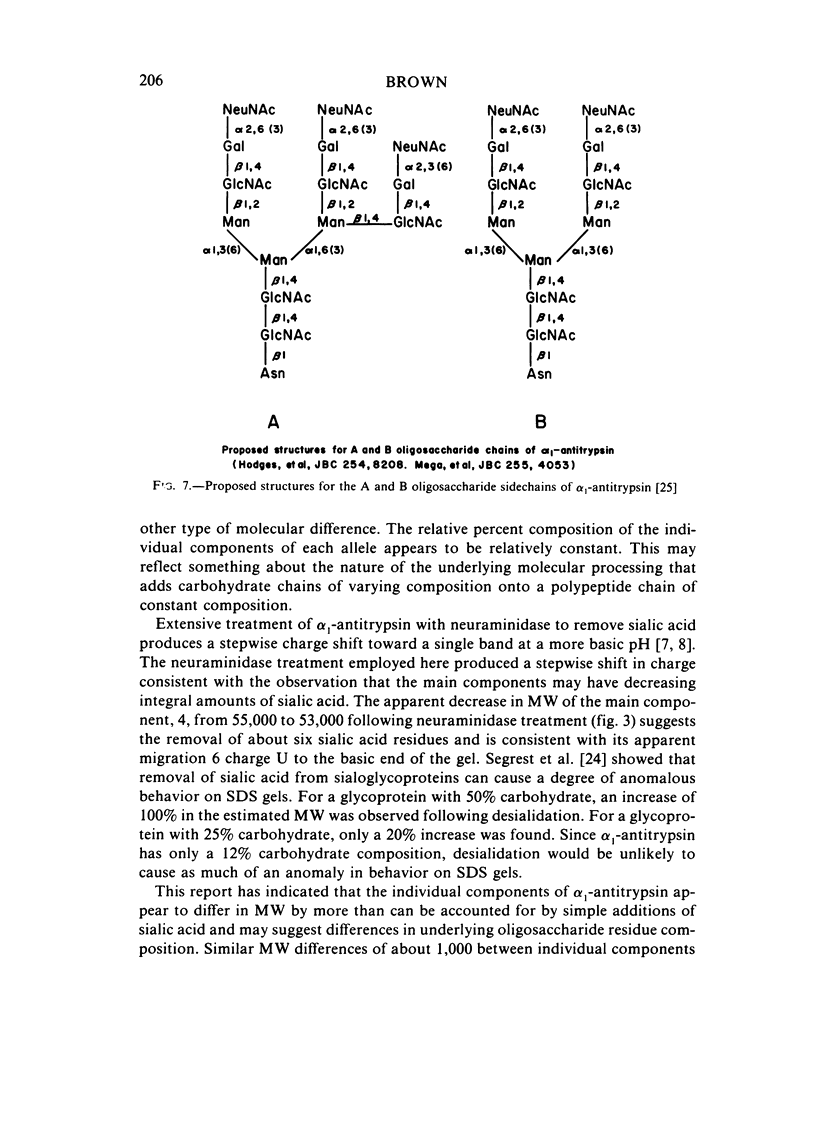
Abstract
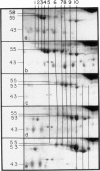
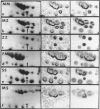
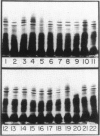
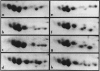
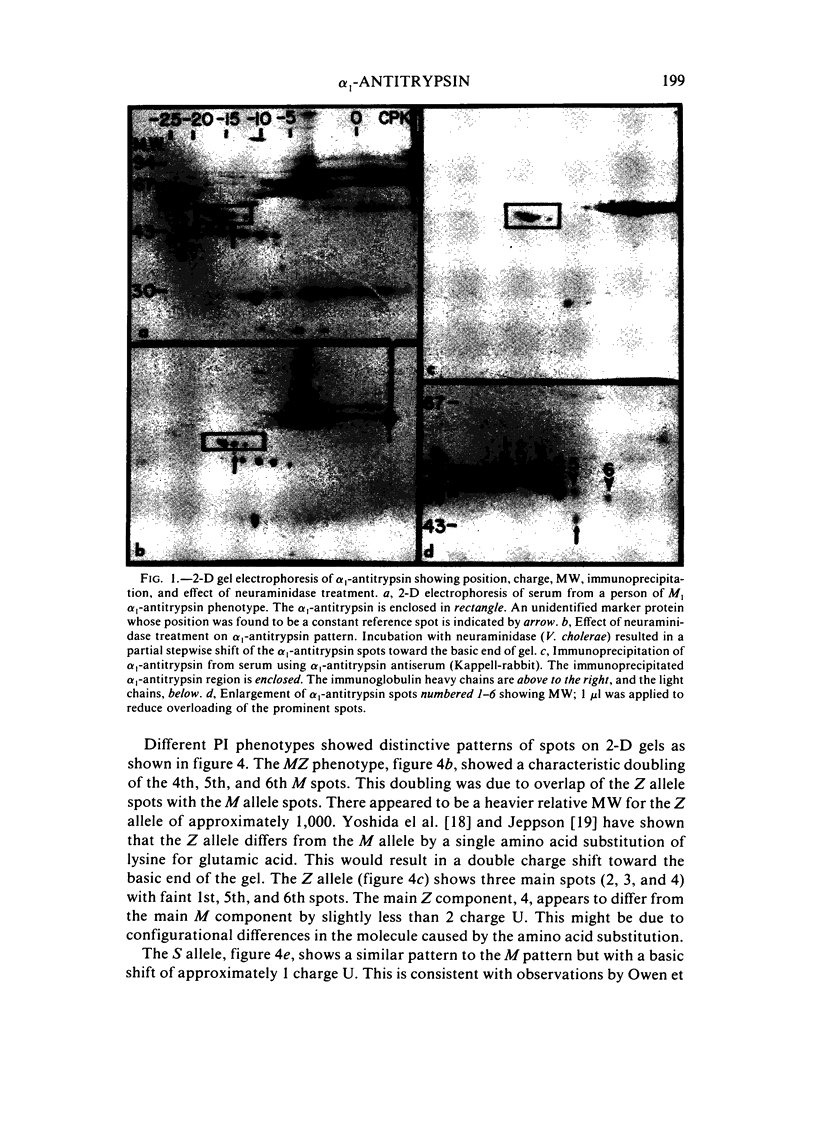
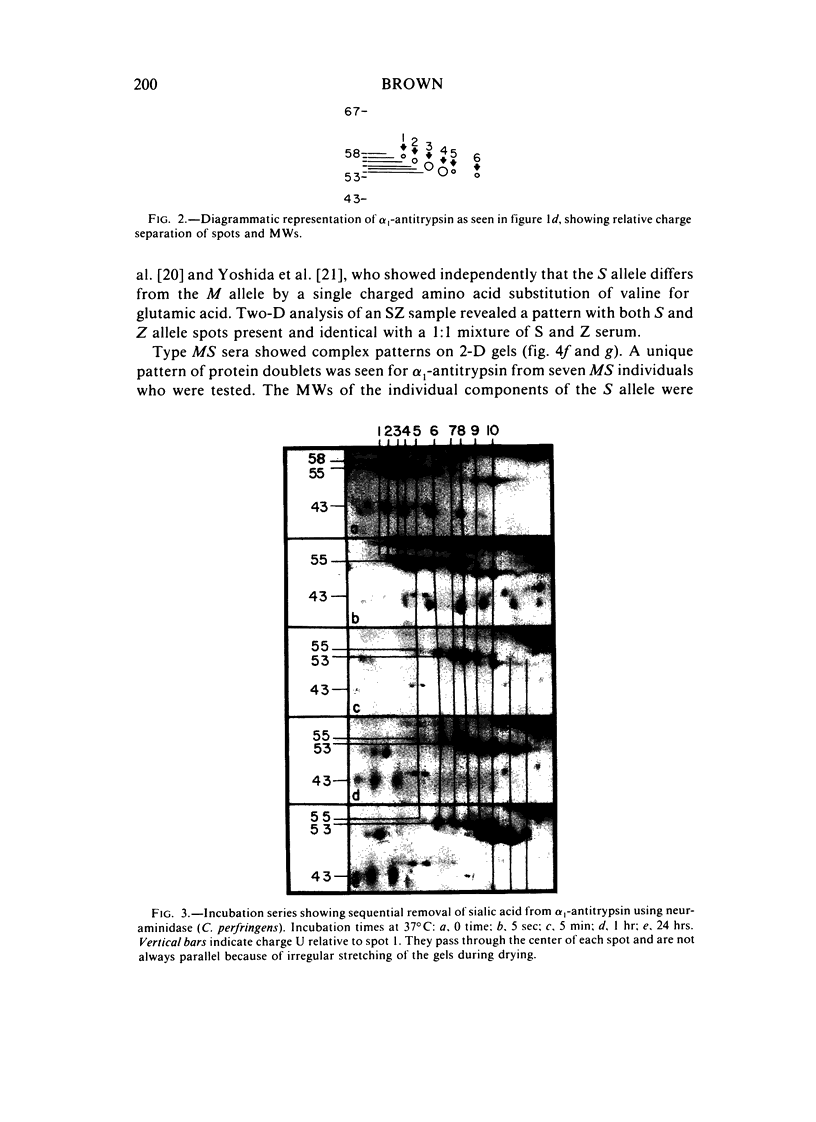
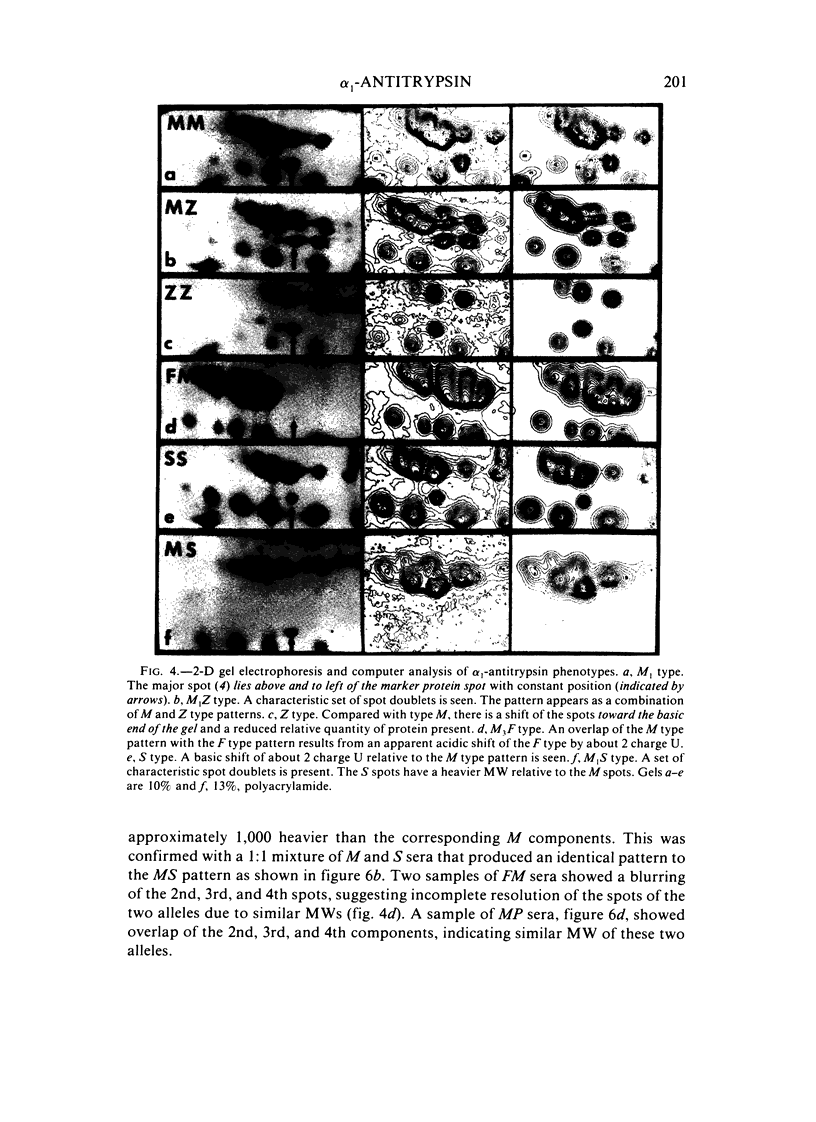
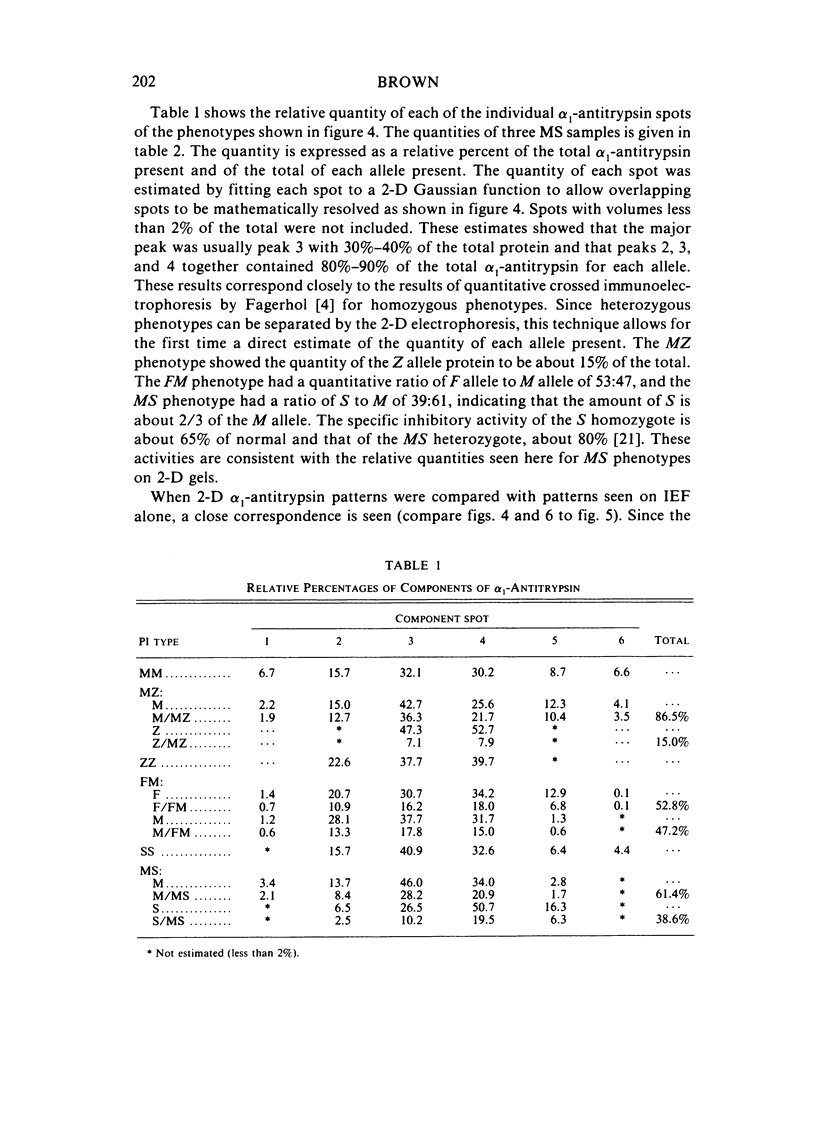
Two-dimensional (2-D) gel electrophoresis was used to examine charge and molecular weight variability of alpha 1-antitrypsin. Two-D electrophoresis resolved distinctive differences among individual phenotypes. Microheterogeneity of charge was seen for the different alleles that corresponded to the charge variability observed on isoelectric focusing gels. The molecular weights of the major components of each allele appeared to differ from each other by approximately 1,000, suggesting, that in addition to sialic acid, there may be differences in neutral sugar composition between the individual components. In comparison to the M allele components, the corresponding S and Z components had higher molecular weights. The MZ and MS phenotypes showed characteristic patterns of protein spot doublets. Computerized quantitation was used to separate and estimate the contribution of each component to the overall allele composition. The Z allele components contained about 15% of the total MZ quantity. The 2-D electrophoresis technique may offer a new approach for molecular structural studies of alpha 1-antitrypsin variants and similar glycoproteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Anderson N. G. Microheterogeneity of serum transferrin, haptoglobin and alpha 2 HS glycoprotein examined by high resolution two-dimensional electrophoresis. Biochem Biophys Res Commun. 1979 May 14;88(1):258–265. doi: 10.1016/0006-291x(79)91724-8. [DOI] [PubMed] [Google Scholar]
- Anderson N. L., Hickman B. J. Analytical techniques for cell fractions. XXIV. Isoelectric point stadnards for two-dimensional electrophoresis. Anal Biochem. 1979 Mar;93(2):312–320. doi: 10.1016/s0003-2697(79)80157-8. [DOI] [PubMed] [Google Scholar]
- Berninger R. W., Talamo R. C. Preparation of concanavalin A free purified alpha-1-antitrypsin. Anal Biochem. 1979 Feb;93(1):180–188. [PubMed] [Google Scholar]
- Comings D. E., Peters K. E. Triple-spot proteins in two-dimensional gel electrophoresis. Am J Hum Genet. 1979 May;31(3):311–314. [PMC free article] [PubMed] [Google Scholar]
- Cox D. W. Genetic variation of alpha 1-antitrypsin. Am J Hum Genet. 1978 Nov;30(6):660–662. [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Purification and properties of normal human alpha 1-antitrypsin. Arch Biochem Biophys. 1973 May;156(1):215–222. doi: 10.1016/0003-9861(73)90359-7. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K. Quantitative studies on the inherited variants of serum alpha-1-antitrypsin. Scand J Clin Lab Invest. 1969 Feb;23(1):97–103. doi: 10.3109/00365516909078092. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Hodges L. C., Laine R., Chan S. K. Structure of the oligosaccharide chains in human alpha 1-protease inhibitor. J Biol Chem. 1979 Sep 10;254(17):8208–8212. [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Fagerhol M. Properties of isolated human alpha1-antitrypsins of Pi types M, S and Z. Eur J Biochem. 1978 Feb 1;83(1):143–153. doi: 10.1111/j.1432-1033.1978.tb12078.x. [DOI] [PubMed] [Google Scholar]
- Kueppers F., Christopherson M. J. Alpha1-antitrypsin: further genetic heterogeneity revealed by isoelectric focusing. Am J Hum Genet. 1978 Jul;30(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- Kueppers F. Determination of alpha1-antitrypsin phenotypes by isoelectric focusing in polyacrylamide gels. J Lab Clin Med. 1976 Jul;88(1):151–155. [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. I. Isolation of glycopeptides. J Biol Chem. 1980 May 10;255(9):4053–4056. [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. II. Structure of oligosaccharides. J Biol Chem. 1980 May 10;255(9):4057–4061. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pierce J. A., Eradio B., Dew T. A. Antitrypsin phenotypes in St. Louis. JAMA. 1975 Feb 10;231(6):609–612. [PubMed] [Google Scholar]
- Roll D., Aguanno J. J., Coffee C. F., Glew R. H., Iammarino R. M. Comparison of the carbohydrate and amino acid composition of normal and S-variant alpha-1-antitrypsin. Biochim Biophys Acta. 1978 Jan 25;532(1):171–178. doi: 10.1016/0005-2795(78)90460-9. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Ewing C., Wessels M., Lieberman J., Gaidulis L. Molecular abnormality of PI S variant of human alpha1-antitrypsin. Am J Hum Genet. 1977 May;29(3):233–239. [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Lieberman J., Gaidulis L., Ewing C. Molecular abnormality of human alpha1-antitrypsin variant (Pi-ZZ) associated with plasma activity deficiency. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1324–1328. doi: 10.1073/pnas.73.4.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Wessels M. Origin of the multiple components of human alpha1-antitrypsin. Biochem Genet. 1978 Aug;16(7-8):641–649. doi: 10.1007/BF00484720. [DOI] [PubMed] [Google Scholar]