Abstract
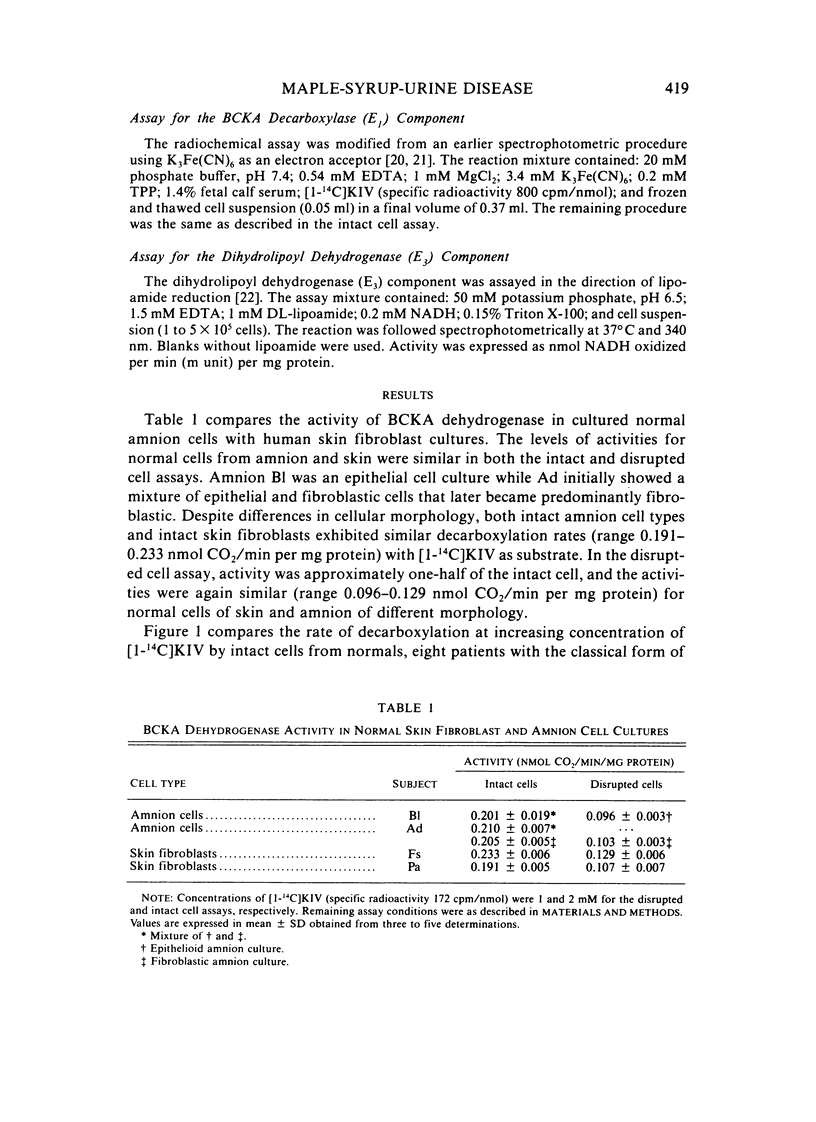
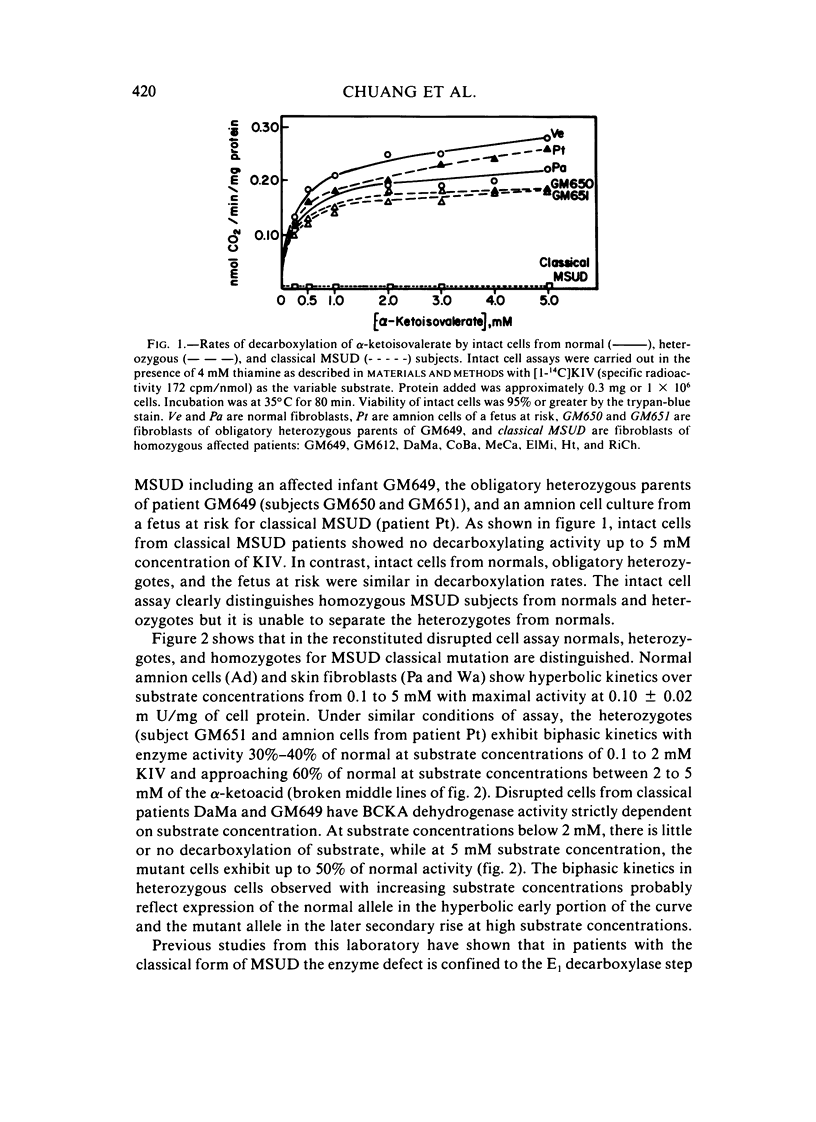
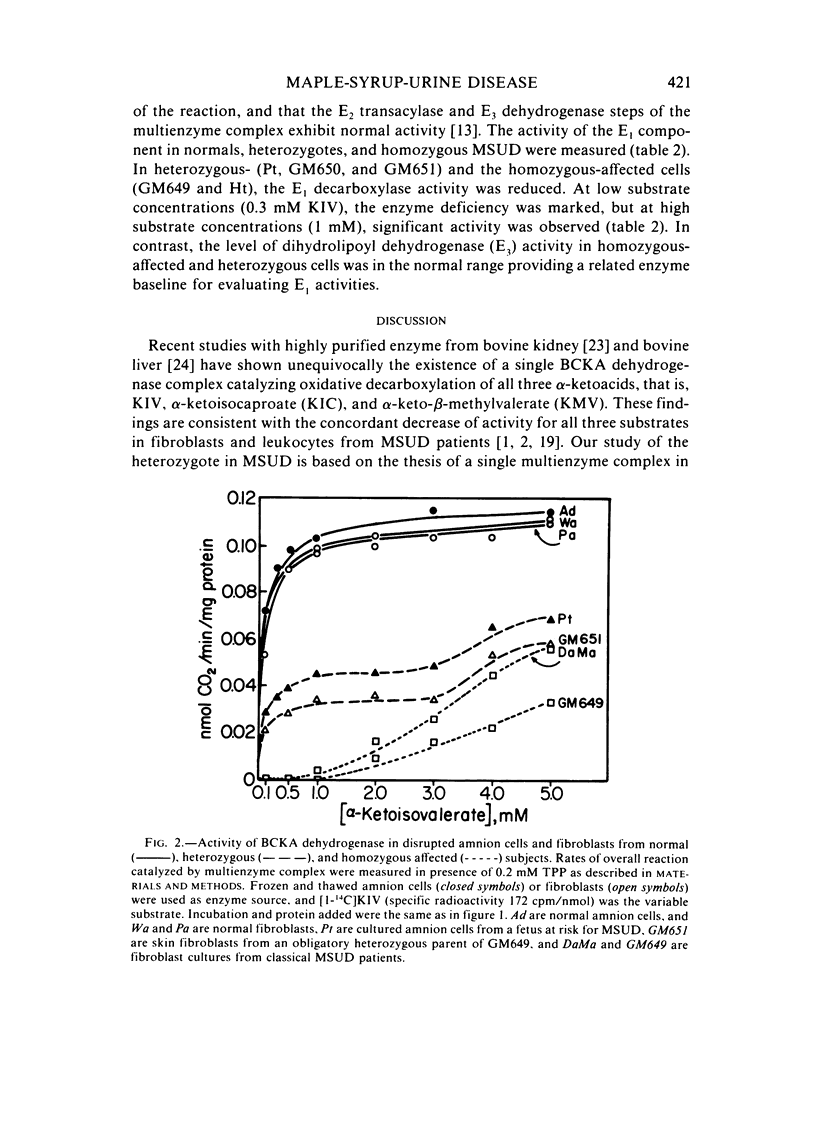
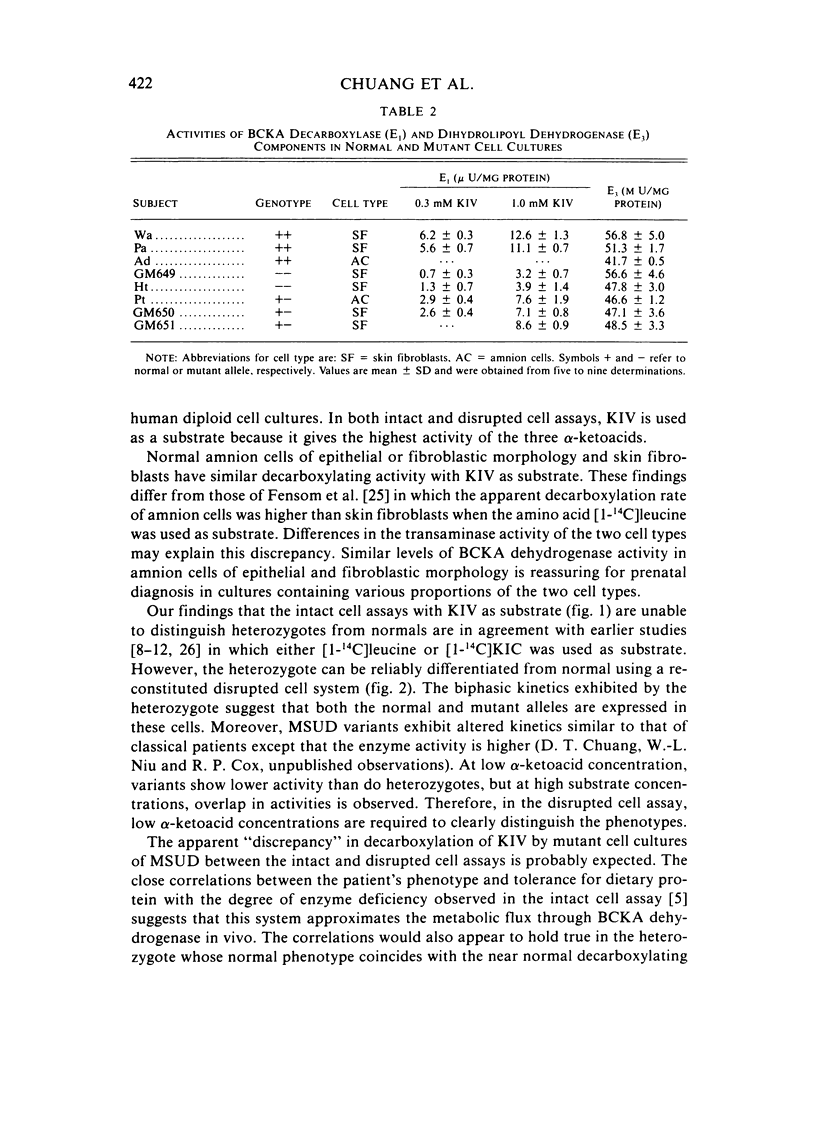
To detect heterozygotes for maple-syrup-urine disease (MSUD), activities of branched-chain-alpha-ketoacid (BCKA) dehydrogenase and its components in skin fibroblasts of two obligatory heterozygotes and amnion cells of a fetus at risk were measured. Intact heterozygous cells were found to decarboxylate [1-14C] alpha-ketoisovalerate at rates equal to or only slightly lower than normal subjects. The inability to differentiate heterozygotes from normals with the intact cell assay confirms earlier studies with intact leukocytes using [1-14C]leucine as substrate. By contrast, measurements of BCKA dehydrogenase activity with disrupted cell suspensions showed MSUD heterozygotes with 30%--60% of normal activity. Moreover, biphasic kinetics in heterozygous cells were observed with increasing substrate concentrations. The altered biphasic kinetics probably reflect expression of the normal allele in the early hyperbolic portion of the curve of the mutant allele in the later secondary rise at high substrate concentrations. Assays of component activities showed concordant E1 decarboxylase deficiency in both heterozygous- and homozygous-affected cells, whereas the E3, dihydrolipoyl dehydrogenase-component, activity was normal. The above results taken together appear to provide an approach to detection of the heterozygote in MSUD.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CIECIURA S. J., MARCUS P. I., PUCK T. T. Clonal growth in vitro of epithelial cells from normal human tissues. J Exp Med. 1956 Oct 1;104(4):615–628. doi: 10.1084/jem.104.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D. T., Niu W. L., Cox R. P. Activities of branched-chain 2-oxo acid dehydrogenase and its components in skin fibroblasts from normal and classical-maple-syrup-urine-disease subjects. Biochem J. 1981 Oct 15;200(1):59–67. doi: 10.1042/bj2000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCIS J., HUTZLER J., LEVITZ M. DETECTION OF THE HETEROZYGOTE IN MAPLE SYRUP URINE DISEASE. J Pediatr. 1965 Mar;66:595–603. doi: 10.1016/s0022-3476(65)80123-8. [DOI] [PubMed] [Google Scholar]
- DANCIS J., HUTZLER J., LEVITZ M. THE DIAGNOSIS OF MAPLE SYRUP URINE DISEASE (BRANCHED- CHAIN KETOACIDURIA) BY THE IN VITRO STUDY OF THE PERIPHERAL LEUKOCYTE. Pediatrics. 1963 Aug;32:234–238. [PubMed] [Google Scholar]
- DANCIS J., JANSEN V., HUTZLER J., LEVITZ M. THE METABOLISM OF LEUCINE IN TISSUE CULTURE OF SKIN FIBROBLASTS OF MAPLE-SYRUP-URINE DISEASE. Biochim Biophys Acta. 1963 Nov 8;77:523–524. doi: 10.1016/0006-3002(63)90536-5. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Cox R. P. Maple syrup urine disease: branched-chain keto acid decarboxylation in fibroblasts as measured with amino acids and keto acids. Am J Hum Genet. 1977 May;29(3):272–279. [PMC free article] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Snyderman S. E., Cox R. P. Enzyme activity in classical and variant forms of maple syrup urine disease. J Pediatr. 1972 Aug;81(2):312–320. doi: 10.1016/s0022-3476(72)80301-9. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Elsas L. J., 2nd Subcellular distribution and cofactor function of human branched chain alpha-ketoacid dehydrogenase in normal and mutant cultured skin fibroblasts. Biochem Med. 1975 May;13(1):7–22. doi: 10.1016/0006-2944(75)90135-0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Elsas L. J., Priest J. H., Wheeler F. B., Danner D. J., Pask B. A. Maple syrup urine disease: coenzyme function and prenatal monitoring. Metabolism. 1974 Jun;23(6):569–579. doi: 10.1016/0026-0495(74)90085-7. [DOI] [PubMed] [Google Scholar]
- Fensom A. H., Benson P. F., Baker J. E. A rapid method for assay of branched-chain keto acid decarboxylation in cultured cells and its application to prenatal diagnosis of maple syrup urine disease. Clin Chim Acta. 1978 Jul 1;87(1):169–174. doi: 10.1016/0009-8981(78)90072-4. [DOI] [PubMed] [Google Scholar]
- GUBLER C. J. Studies on the physiological functions of thiamine. I. The effects of thiamine deficiency and thiamine antagonists on the oxidation of alpha-keto acids by rat tissues. J Biol Chem. 1961 Dec;236:3112–3120. [PubMed] [Google Scholar]
- Goedde H. W., Langenbeck U., Brackertz D. Detection of heterozygotes in maple syrup urin disease: role of lymphocyte count. Humangenetik. 1968;6(2):189–190. doi: 10.1007/BF00297728. [DOI] [PubMed] [Google Scholar]
- Koike M., Koike K. Structure, assembly and function of mammalian alpha-keto acid dehydrogenase complexes. Adv Biophys. 1976:187–227. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langenbeck U., Rüdiger H. W., Schulze-Schencking M., Keller W., Brackertz D., Goedde H. W. Evaluation of a heterozygote test for maple syrup urine disease in leucocytes and cultured fibroblasts. Humangenetik. 1971;11(4):304–315. doi: 10.1007/BF00278658. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Cox R. P., Dancis J. Complementation analysis of maple syrup urine disease in heterokaryons derived from cultured human fibroblasts. Nature. 1973 Jun 29;243(5409):533–535. doi: 10.1038/243533a0. [DOI] [PubMed] [Google Scholar]
- McKnight M. T., Spence M. W. Attempted detection of heterozygotes for maple-syrup-urine disease. Clin Genet. 1972;3(6):458–464. doi: 10.1111/j.1399-0004.1972.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Kahler S. G., Kirkman H. N. Lactic acidemia, neurologic deterioration and carbohydrate dependence in a girl with dihydrolipoyl dehydrogenase deficiency. Eur J Pediatr. 1981 Mar;136(1):35–39. doi: 10.1007/BF00441708. [DOI] [PubMed] [Google Scholar]
- Stumpf D. A., Parks J. K. Friedreich ataxia. II. Normal kinetics of lipoamide dehydrogenase. Neurology. 1979 Jun;29(6):820–826. doi: 10.1212/wnl.29.6.820. [DOI] [PubMed] [Google Scholar]
- Sullivan S. G., Dancis J., Cox R. P. Modulation of branched-chain alpha-keto acid decarboxylase activity in rat liver mitochondria by hypophysectomy. Arch Biochem Biophys. 1976 Sep;176(1):225–234. doi: 10.1016/0003-9861(76)90160-0. [DOI] [PubMed] [Google Scholar]
- Wendel U., Wentrup H., Rüdiger H. W. Maple syrup urine disease: analysis of branched chain ketoacid decarboxylation in cultured fibroblasts. Pediatr Res. 1975 Sep;9(9):709–717. doi: 10.1203/00006450-197509000-00005. [DOI] [PubMed] [Google Scholar]
- Wendel U., Wöhler W., Goedde H. W., Langenbeck U., Passarge E., Rüdiger H. W. Rapid diagnosis of maple syrup urine disease (branched chain ketoaciduria) by micro-enzyme assay in leukocytes and fibroblasts. Clin Chim Acta. 1973 May 30;45(4):433–440. doi: 10.1016/0009-8981(73)90046-6. [DOI] [PubMed] [Google Scholar]


