Abstract
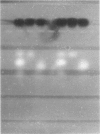
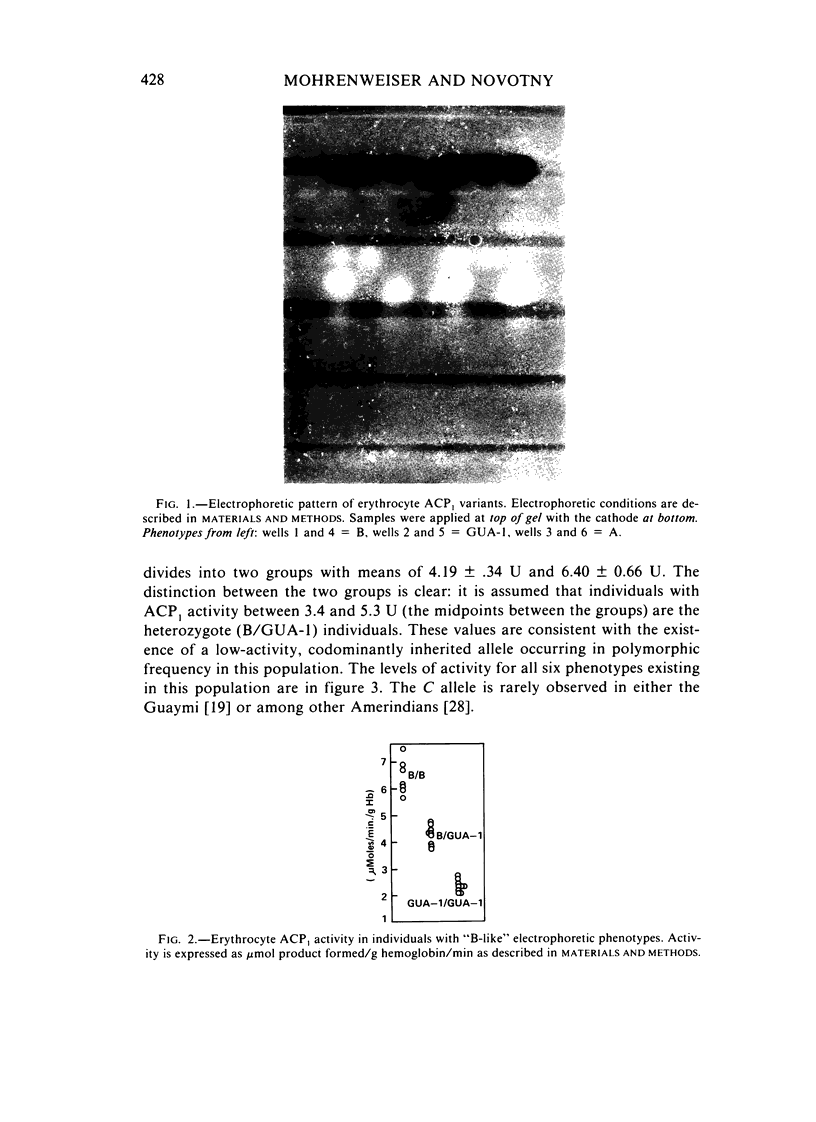
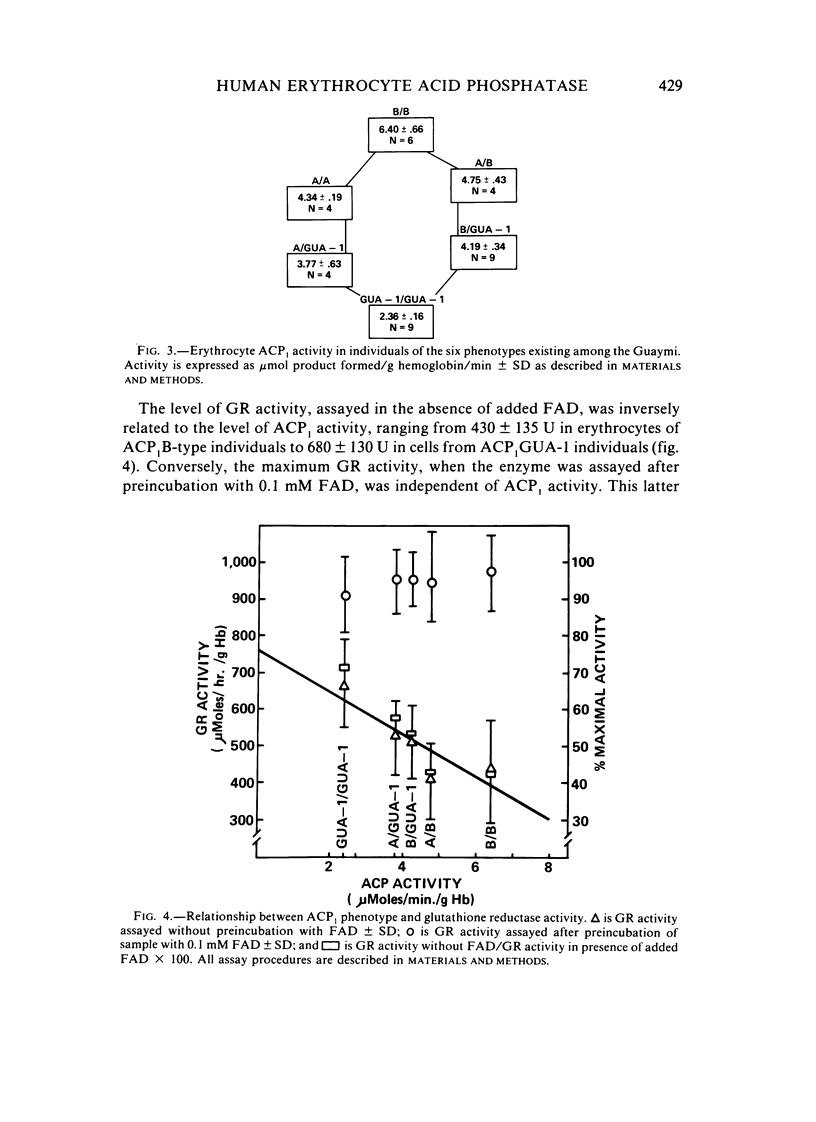
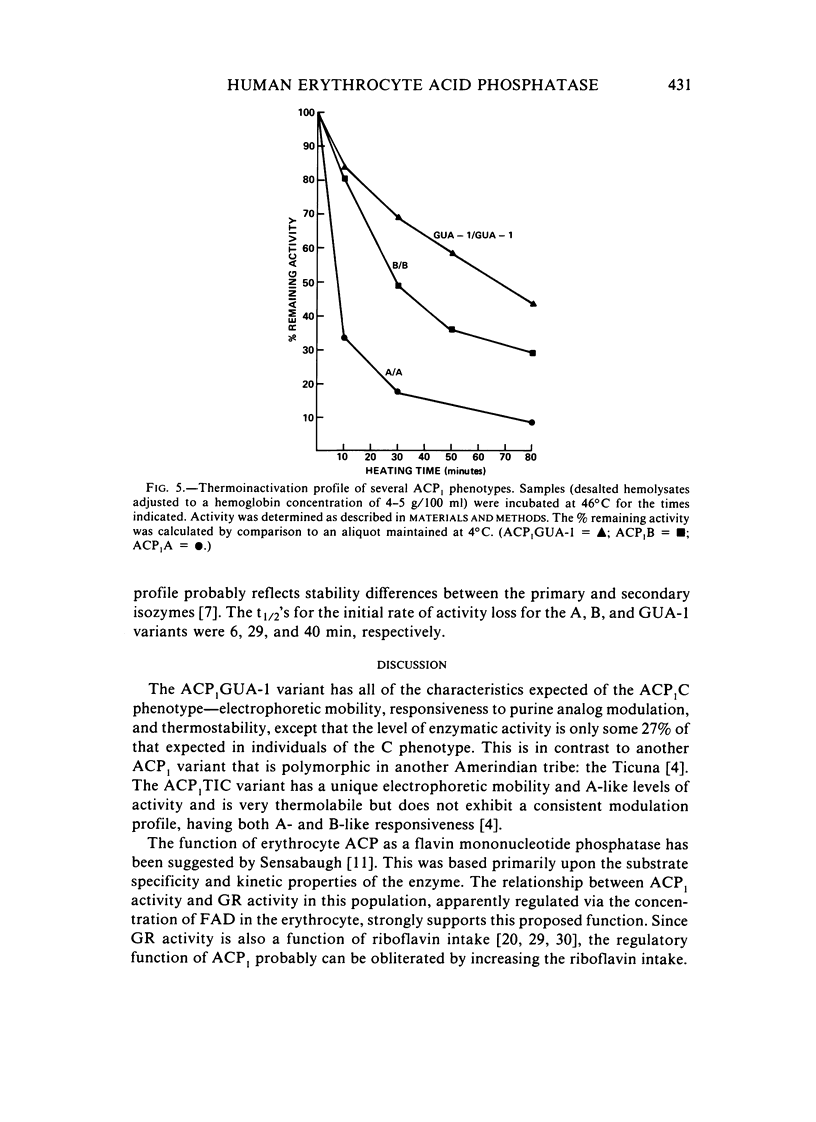
ACP1GUA-1, a variant of human erythrocyte acid phosphatase, exists as a polymorphism (allele frequency of .132) in the Guaymi Indians of Central America. This variant has an electrophoretic mobility similar to the common B- and C-type variants, but individuals of the ACP1GUA-1 phenotype have a level of enzyme activity only 27% of the activity expected for the ACP1C variant. The GUA-1 variant is more thermostable than is the B variant, and the order of responsiveness to the modulation of activity by purine analogs and folate is always (B)-(A)-(GUA-1). Thus, the GUA-1 variant is a low-activity variant with C-like regulatory properties. Erythrocytes from individuals of the ACP1GUA-1 phenotype have increased basal levels of glutathione reductase, and a larger fraction of the glutathione reductase protein is present as the holoenzyme, indicating increased levels of flavin adenine dinucleotide in the erythrocytes of these individuals. This is consistent with the suggestion that ACP1 has a physiological function as a flavin mononucleotide phosphatase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottini E., Carapella E., Orzalezi M., Lucarelli P., Pascone R., Gloria-Bottini F., Coccia M. Is there a role of erythrocyte acid phosphatase polymorphism in intrauterine development? Am J Hum Genet. 1980 Sep;32(5):764–767. [PMC free article] [PubMed] [Google Scholar]
- Bottini E., Lucarelli P., Agostino R., Palmarino R., Businco L., Antognoni G. Favism: association with erythrocyte acid phosphatase phenotype. Science. 1971 Jan 29;171(3969):409–411. doi: 10.1126/science.171.3969.409. [DOI] [PubMed] [Google Scholar]
- Bottini E., Lucarelli P., Bastianon V., Gloria F. Erythrocyte acid phosphatase polymorphism and haemolysis. J Med Genet. 1972 Dec;9(4):434–435. doi: 10.1136/jmg.9.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini E., Scacchi R., Gloria-Bottini F., Mortera J., Palmarino R., Carapella-De-Luca E., Lapi A. S., Nodari C. Letter: Neonatal jaundice and erythrocyte-acid-phosphatase phenotype. Lancet. 1976 Apr 24;1(7965):918–918. doi: 10.1016/s0140-6736(76)92148-6. [DOI] [PubMed] [Google Scholar]
- Brewster M. A., Berry D. H., Murphey M. N. Automated reaction rate analysis of erythrocyte glucose-6-phosphate dehydrogenase and glutathione reductase activities. Biochem Med. 1974 Jul;10(3):229–235. doi: 10.1016/0006-2944(74)90026-x. [DOI] [PubMed] [Google Scholar]
- Carapella E., Pascone R., Gori M. G., Matteucci P., Gloria-Bottini F., Mortera J., Lucarelli P., Scacchi R., Bottini E. The genetic component of quantitative perinatal variables. An analysis of relations between erythrocyte acid phosphatase phenotype and birth weight, gestational age and serum bilirubin level in the first days of life. J Perinat Med. 1980;8(1):42–47. [PubMed] [Google Scholar]
- Eze L. C., Tweedie M. C., Bullen M. F., Wren P. J., Evans D. A. Quantitative genetics of human red cell acid phosphatase. Ann Hum Genet. 1974 Jan;37(3):333–340. doi: 10.1111/j.1469-1809.1974.tb01840.x. [DOI] [PubMed] [Google Scholar]
- Fielek S., Mohrenweiser H. W. Erythrocyte enzyme deficiencies assessed with a miniature centrifugal analyzer. Clin Chem. 1979 Mar;25(3):384–388. [PubMed] [Google Scholar]
- Fisher R. A., Harris H. Studies on the separate isozymes of red cell acid phosphatase phenotypes A and B. II. Comparison of kinetics and stabilities of the isozymes. Ann Hum Genet. 1971 May;34(4):439–448. doi: 10.1111/j.1469-1809.1971.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Glatzle D., Vuilleumier J. P., Weber F., Decker K. Glutathione reductase test with whole blood, a convenient procedure for the assessment of the riboflavin status in humans. Experientia. 1974 Jun 15;30(6):665–667. doi: 10.1007/BF01921531. [DOI] [PubMed] [Google Scholar]
- HOPKINSON D. A., SPENCER N., HARRIS H. GENETICAL STUDIES ON HUMAN RED CELL ACID PHOSPHATASE. Am J Hum Genet. 1964 Mar;16:141–154. [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst E., Stokes G. B. Microcentrifuge desalting: a rapid, quantitative method for desalting small amounts of protein. Anal Biochem. 1980 May 1;104(1):130–135. doi: 10.1016/0003-2697(80)90287-0. [DOI] [PubMed] [Google Scholar]
- Hoppe H. H., Hennig W., Brinkmann B. Horizontal polyacrylamide electrophoresis for the determination of serum protein (haptoglobin) and red cell enzyme polymorphisms. Humangenetik. 1972;14(3):224–231. doi: 10.1007/BF00278041. [DOI] [PubMed] [Google Scholar]
- Jenkins T., Corfield V. The red cell acid phosphatase polymorphism in Southern Africa: population data and studies on the R, RA and RB phenotypes. Ann Hum Genet. 1972 Apr;35(4):379–391. [PubMed] [Google Scholar]
- Luffman J. E., Harris H. A comparison of some properties of human red cell acid phosphatase in different phenotypes. Ann Hum Genet. 1967 May;30(4):387–401. doi: 10.1111/j.1469-1809.1967.tb00040.x. [DOI] [PubMed] [Google Scholar]
- Mansfield E., Sensabaugh G. F. Phenotypic differences in purine modulation of erythrocyte acid-phosphatase activity. Lancet. 1977 Jul 23;2(8030):201–202. doi: 10.1016/s0140-6736(77)90225-2. [DOI] [PubMed] [Google Scholar]
- Mansfield E., Sensabaugh G. F. Red cell acid phosphatase: modulation of activity by purines. Prog Clin Biol Res. 1978;21:233–249. [PubMed] [Google Scholar]
- Mohrenweiser H. W., Fielek S., Wurzinger K. H. Characteristics of enzymes of erythrocytes from newborn infants and adults: activity, thermostability, and electrophoretic profile as a function of cell age. Am J Hematol. 1981 Sep;11(2):125–136. doi: 10.1002/ajh.2830110203. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrenweiser H. W., Neel J. V. Frequency of thermostability variants: estimation of total "rare" variant frequency in human populations. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5729–5733. doi: 10.1073/pnas.78.9.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Mohrenweiser H. W., Meisler M. H. Rate of spontaneous mutation at human loci encoding protein structure. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6037–6041. doi: 10.1073/pnas.77.10.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. Rare variants, private polymorphisms, and locus heterozygosity in Amerindian populations. Am J Hum Genet. 1978 Sep;30(5):465–490. [PMC free article] [PubMed] [Google Scholar]
- Nichoalds G. E. Assessment of status riboflavin nutriture by assay of erythrocyte glutathione reductase activity. Clin Chem. 1974 May;20(5):624–628. [PubMed] [Google Scholar]
- Oski F. A., Komazawa M. Metabolism of the erythrocytes of the newborn infant. Semin Hematol. 1975 Apr;12(2):209–221. [PubMed] [Google Scholar]
- SPENCER N., HOPKINSON D. A., HARRIS H. QUANTITATIVE DIFFERENCES AND GENE DOSAGE IN THE HUMAN RED CELL ACID PHOSPHATASE POLYMORPHISM. Nature. 1964 Jan 18;201:299–300. doi: 10.1038/201299a0. [DOI] [PubMed] [Google Scholar]
- Sensabaugh G. F., Golden V. L. Phenotype dependence in the inhibition of red cell acid phosphatase (ACP) by folates. Am J Hum Genet. 1978 Sep;30(5):553–560. [PMC free article] [PubMed] [Google Scholar]
- Tanis R. J., Neel J. V., Torres de Arauz R. Two more "private" polymorphisms of Amerindian tribes: LDHb GUA-1 and ACP1 B GUA-1 in the Guaymi in Panama. Am J Hum Genet. 1977 Sep;29(5):419–430. [PMC free article] [PubMed] [Google Scholar]
- VALENTINE W. N., TANAKA K. R., FREDRICKS R. E. Erythrocyte acid phosphatase in health and disease. Am J Clin Pathol. 1961 Oct;36:328–332. doi: 10.1093/ajcp/36.4.328. [DOI] [PubMed] [Google Scholar]
- Yoshihara C. M., Mohrenweiser H. W. Characterization of ACP1TIC-1, an electrophoretic variant of erythrocyte acid phosphatase restricted to the Ticuna Indians of central Amazonas. Am J Hum Genet. 1980 Nov;32(6):898–907. [PMC free article] [PubMed] [Google Scholar]