Abstract
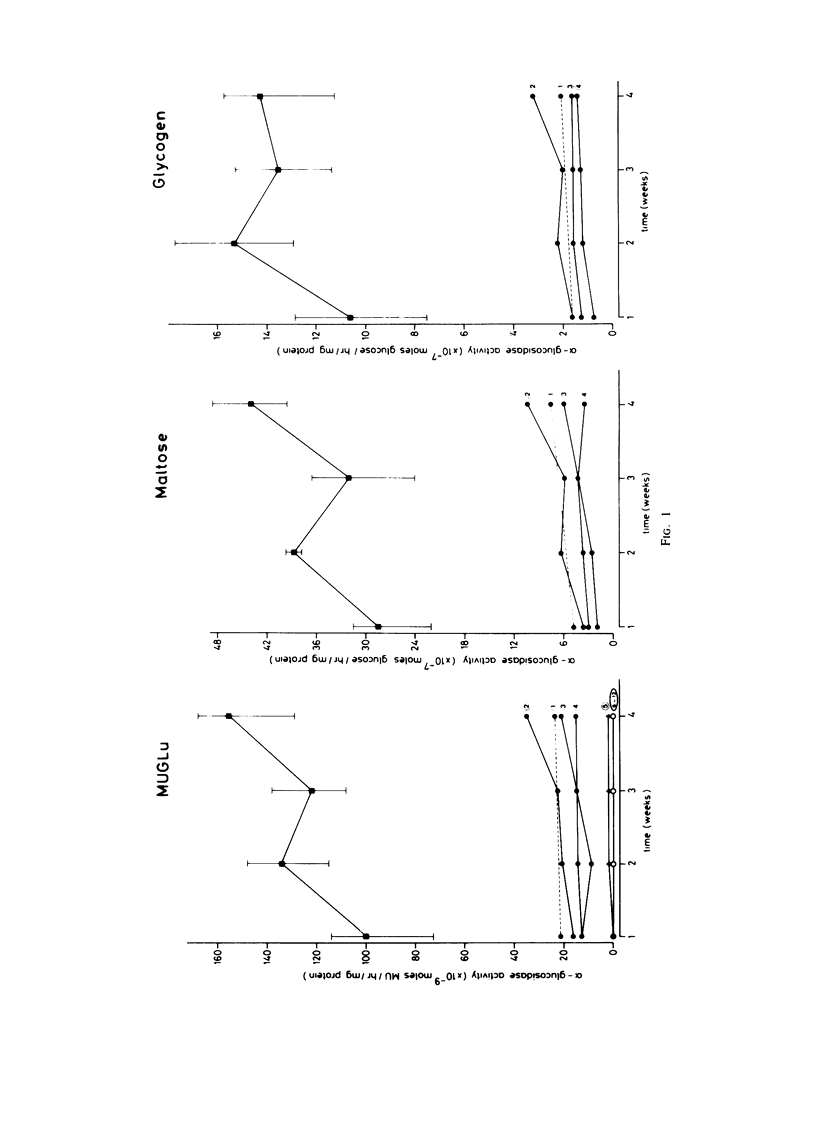
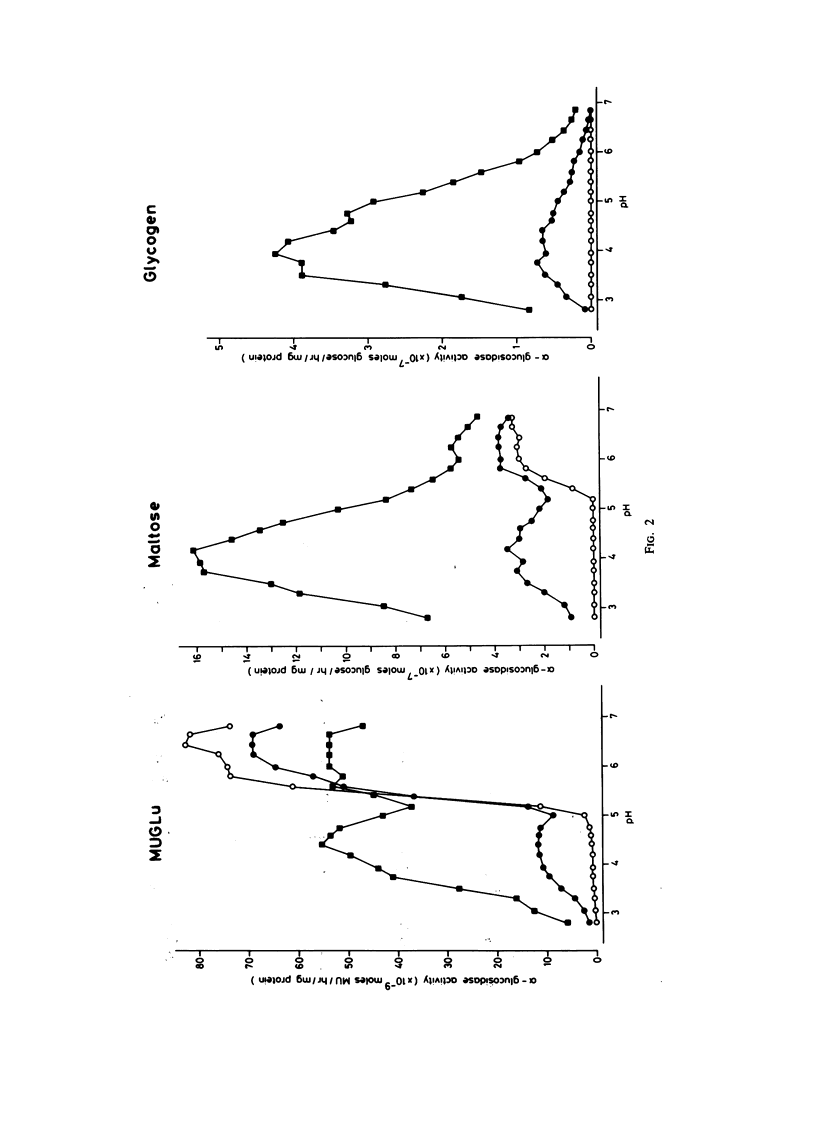
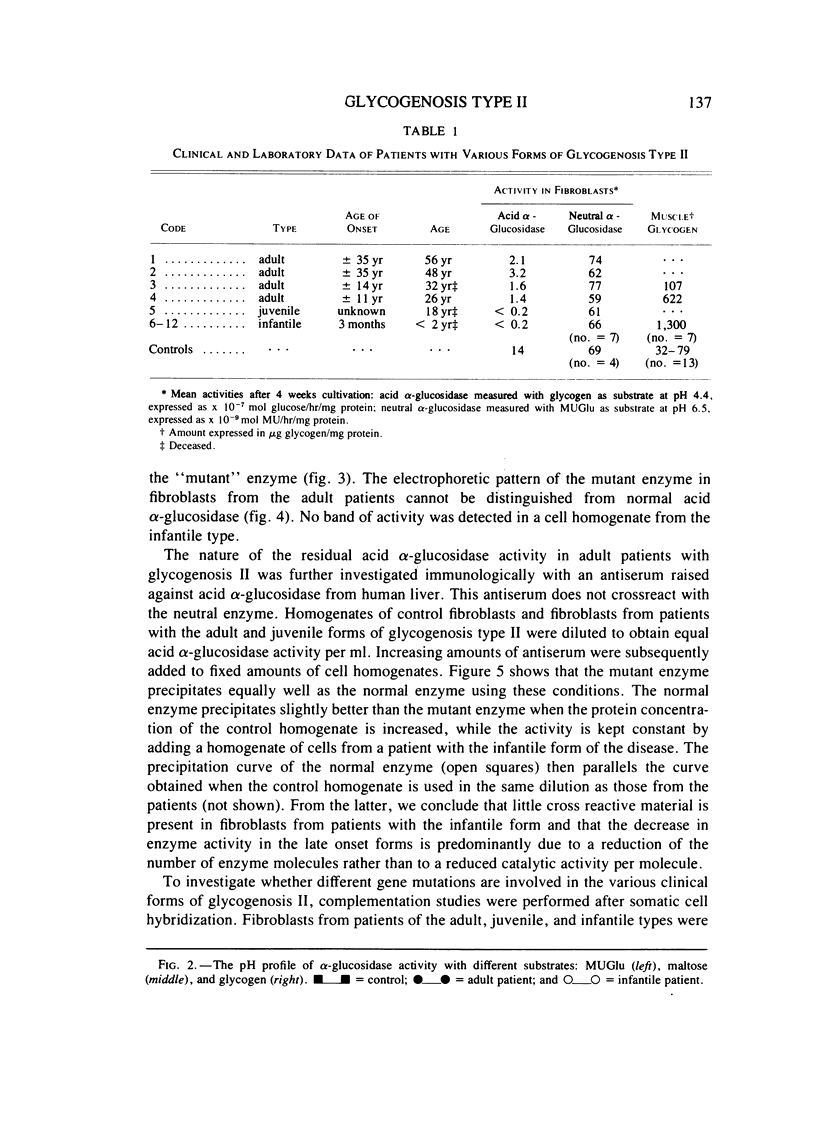
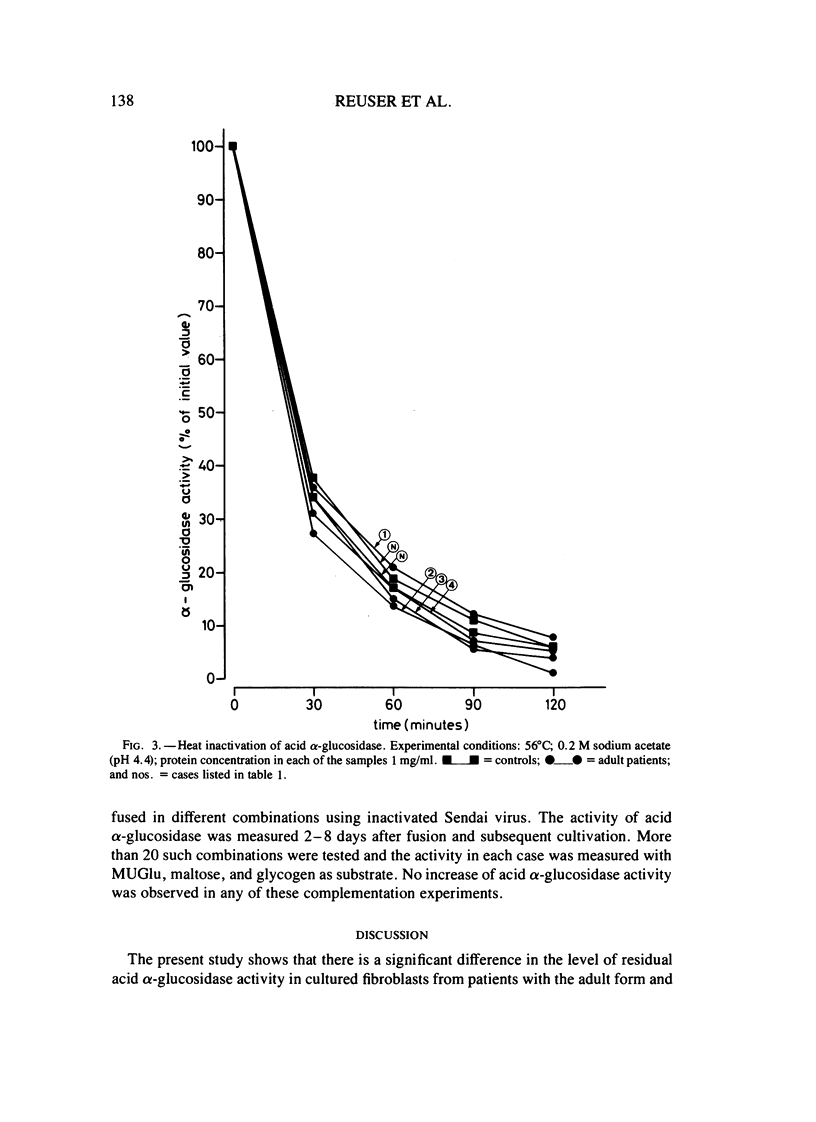
Fibroblasts from patients with the adult, juvenile, and infantile form of glycogenosis type II (Pompe disease) were cultured under standardized conditions, and the activity of acid alpha-glucosidase (E.C.3.2.1.20) towards glycogen, maltose, and 4-methylumbelliferyl-alpha-D-glucopyranoside was measured. Glycogen levels in muscle biopsies and in cultured fibroblasts from patients were determined. Residual enzyme activities varying from 7%-22% were detected in fibroblasts from patients with the adult form but not from patients with the infantile form of glycogenosis II. An inverse correlation was found between the severity of the clinical manifestation and the degree of residual enzyme activity in the fibroblasts. The kinetic and electrophoretic properties of acid alpha-glucosidase in fibroblasts from the adult patients and from control individuals were similar. Immunological studies suggested that the decrease of acid alpha-glucosidase activity is caused by a mutation that affects the production or degradation of the enzyme rather than its catalytic activity. Complementation studies were carried out by fusing fibroblasts from patients with the adult, juvenile, and infantile form of glycogenosis II, but neither conventional assays on multikaryons nor enzyme assays on single binuclear heterokaryons gave any evidence for genetic heterogeneity among these forms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini C., Engel A. G. Comparative study of acid maltase deficiency. Biochemical differences between infantile, childhood, and adult types. Arch Neurol. 1972 Apr;26(4):344–349. doi: 10.1001/archneur.1972.00490100074007. [DOI] [PubMed] [Google Scholar]
- Angelini C., Engel A. G. Subcellular distribution of acid and neutral alpha-glucosidases in normal, acid maltase deficient, and myophosphorylase deficient human skeletal muscle. Arch Biochem Biophys. 1973 May;156(1):350–355. doi: 10.1016/0003-9861(73)90374-3. [DOI] [PubMed] [Google Scholar]
- Engel A. G. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93(3):599–616. doi: 10.1093/brain/93.3.599. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Gomez M. R., Seybold M. E., Lambert E. H. The spectrum and diagnosis of acid maltase deficiency. Neurology. 1973 Jan;23(1):95–106. doi: 10.1212/wnl.23.1.95. [DOI] [PubMed] [Google Scholar]
- Galjaard H., Hoogeveen A., de Wit-Verbeek H. A., Reuser A. J., Ho M. W., Robinson D. Genetic heterogeneity in GM1-gangliosidosis. Nature. 1975 Sep 4;257(5521):60–62. doi: 10.1038/257060a0. [DOI] [PubMed] [Google Scholar]
- Galjaard H., Hoogeveen A., de Wit-Verbeek H. A., Reuser A. J., Keijzer W., Westerveld A., Bootsma D. Tay-Sachs and Sandhoff's disease: intergenic complementation after somatic cell hybridization. Exp Cell Res. 1974 Aug;87(2):444–448. doi: 10.1016/0014-4827(74)90515-1. [DOI] [PubMed] [Google Scholar]
- Galjaard H., Mekes M., Josselin de Jong JE D. E., Niermeijer M. F. A method for rapid prenatal diagnosis of glycogenosis II (Pompe's disease). Clin Chim Acta. 1973 Dec 27;49(3):361–375. doi: 10.1016/0009-8981(73)90234-9. [DOI] [PubMed] [Google Scholar]
- Hudgson P., Fulthorpe J. J. The pathology of type II skeletal muscle glycogenosis. A light and electron-microscopic study. J Pathol. 1975 Jul;116(3):139–147. doi: 10.1002/path.1711160303. [DOI] [PubMed] [Google Scholar]
- Huijing F. A rapid enzymic method for glycogen estimation in very small tissue samples. Clin Chim Acta. 1970 Dec;30(3):567–572. doi: 10.1016/0009-8981(70)90246-9. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G., Hülsmann W. C. The use of leucocytes as an aid in the diagnosis of a variant of glycogen storage disease type II (Pompe's disease). Eur J Clin Invest. 1972 Nov;2(6):467–471. doi: 10.1111/j.1365-2362.1972.tb00678.x. [DOI] [PubMed] [Google Scholar]
- Koster J. F., Slee R. G. Some properties of human liver acid alpha-glucosidase. Biochim Biophys Acta. 1977 May 12;482(1):89–97. doi: 10.1016/0005-2744(77)90357-6. [DOI] [PubMed] [Google Scholar]
- Mehler M., DiMauro S. Residual acid maltase activity in late-onset acid maltase deficiency. Neurology. 1977 Feb;27(2):178–184. doi: 10.1212/wnl.27.2.178. [DOI] [PubMed] [Google Scholar]
- Palmer T. N. The maltase, glucoamylase and transglucosylase activities of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):713–724. doi: 10.1042/bj1240713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuser A. J., Jongkind J. F., Galjaard H. Methods for analysis of acid alpha-1,4-glucosidase activity in single hybrid cells. J Histochem Cytochem. 1976 Apr;24(4):578–586. doi: 10.1177/24.4.1063791. [DOI] [PubMed] [Google Scholar]
- Rosenfeld E. L. Alpha-glucosidases (gamma-amylases) in human and animal organisms. Pathol Biol (Paris) 1975 Jan;23(1):71–84. [PubMed] [Google Scholar]
- Smith J., Zellweger H., Afifi A. K. Muscular form of glycogenosis, type II (Pompe). Neurology. 1967 Jun;17(6):537–549. doi: 10.1212/wnl.17.6.537. [DOI] [PubMed] [Google Scholar]
- Thomas G. H., Taylor H. A., Miller C. S., Axelman J., Migeon B. R. Genetic complementation after fusion of Tay-Sachs and Sandhoff cells. Nature. 1974 Aug 16;250(467):580–582. doi: 10.1038/250580a0. [DOI] [PubMed] [Google Scholar]
- de Barsy T., Jacquemin P., Devos P., Hers H. G. Rodent and human acid -glucosidase. Purification, properties and inhibition by antibodies. Investigation in type II glycogenosis. Eur J Biochem. 1972 Nov 21;31(1):156–165. doi: 10.1111/j.1432-1033.1972.tb02514.x. [DOI] [PubMed] [Google Scholar]



