Abstract
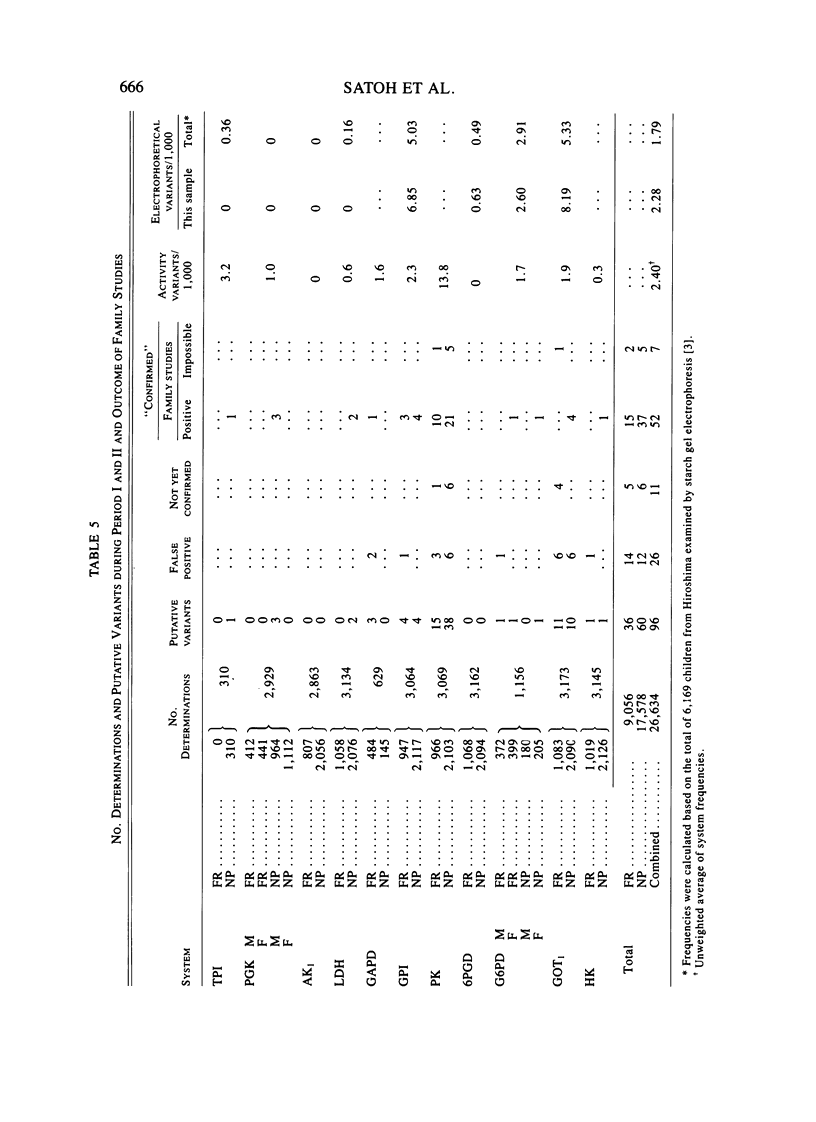
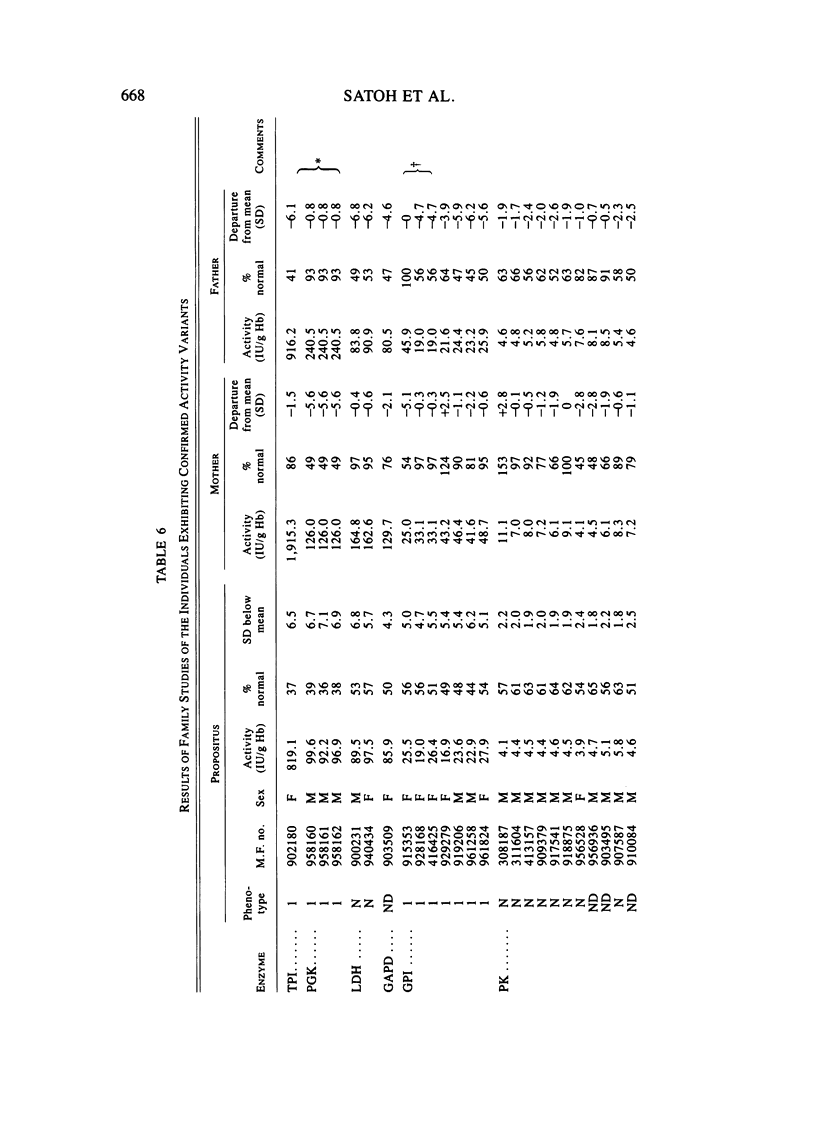
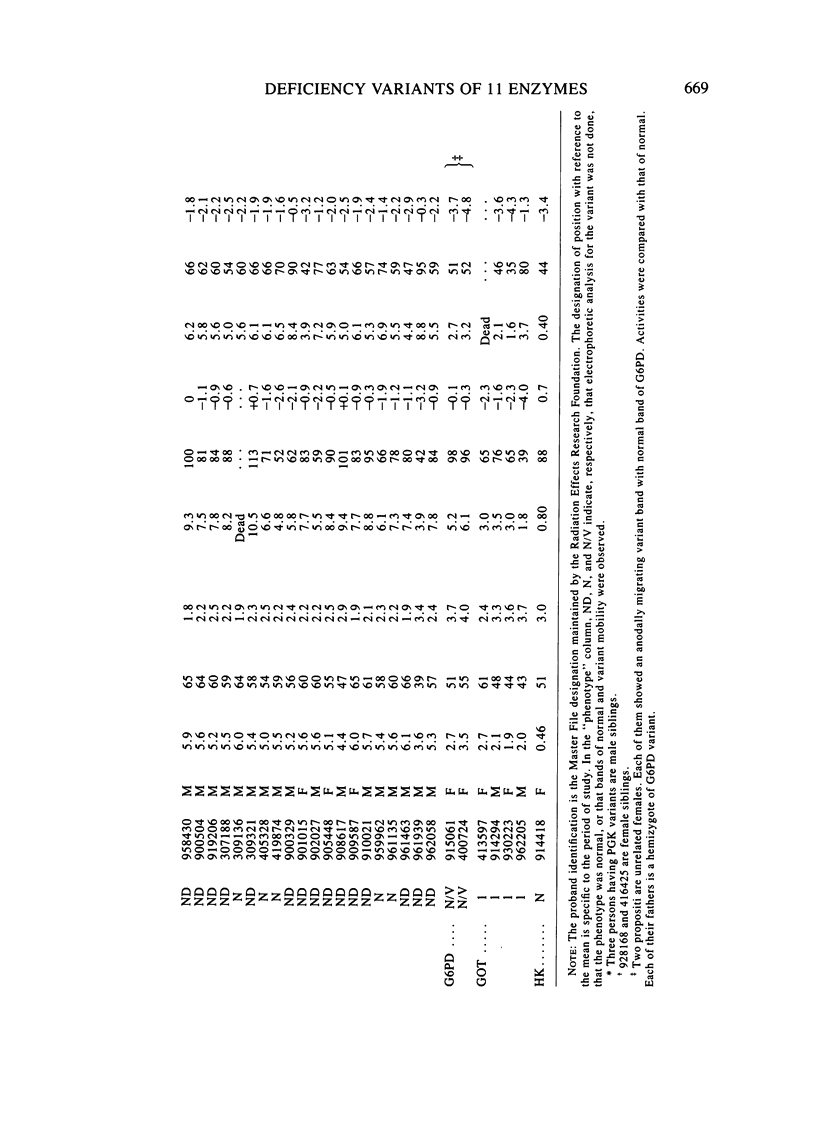
Eleven human enzymes, chosen for this study because of relatively small coefficients of variation for mean activity, have been surveyed for the frequency with which activities less than or equal to 66% of the mean value occur. This criterion should detect almost all heterozygotes for variants lacking any activity plus a fraction of the persons with variants characterized by markedly depressed activity and/or instability. The enzymes surveyed are TPI, PGK, AK1, LDH, GAPD, GPI, PK, 6PGD, G6PD, GOT1, and HK. The number of determinations per enzyme ranged from 310 to 3,173, for a total of 26,634 determinations. Family studies have thus far been possible in 52 instances in which the initial observation of activity less than or equal to 66% of normal was confirmed. In every instance, a parent exhibited a similar finding, giving confidence that a true genetic entity was being detected. With this approach, the frequency of heterozygotes per 1,000 determinations varied from 0.0 (AK1, 6PGD) to 13.8 (PK), with an average of 2.4. For these same systems, in this laboratory the frequency of "rare" electrophoretic variants is 2.3/1,000, the ratio of the latter to the former thus being 1.0 in Japanese. Our experience with these deficiency phenotypes to date suggests that for selected enzymes such phenotypes can be incorporated into a program designed to detect mutational events.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Blume K. G., Kaplan J. C., Löhr G. W., Ramot B., Valentine W. N. International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol. 1977 Feb;35(2):331–340. doi: 10.1111/j.1365-2141.1977.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Fielek S., Mohrenweiser H. W. Erythrocyte enzyme deficiencies assessed with a miniature centrifugal analyzer. Clin Chem. 1979 Mar;25(3):384–388. [PubMed] [Google Scholar]
- HAMILTON H. B., NEEL J. V., KOBARA T. Y., OZAKI K. The frequency in Japan of carriers of the rare "recessive" gene causing acatalasemia. J Clin Invest. 1961 Dec;40:2199–2208. doi: 10.1172/JCI104446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Miwa S., Fujii H., Fujinami N., Takegawa S., Yamato K. Thirteen cases of pyruvate kinase deficiency found in Japan. Am J Hematol. 1981;10(3):239–250. doi: 10.1002/ajh.2830100303. [DOI] [PubMed] [Google Scholar]
- Johnson F. M., Lewis S. E. Electrophoretically detected germinal mutations induced in the mouse by ethylnitrosourea. Proc Natl Acad Sci U S A. 1981 May;78(5):3138–3141. doi: 10.1073/pnas.78.5.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F. M., Roberts G. T., Sharma R. K., Chasalow F., Zweidinger R., Morgan A., Hendren R. W., Lewis S. E. The detection of mutants in mice by electrophoresis: results of a model induction experiment with procarbazine. Genetics. 1981 Jan;97(1):113–124. doi: 10.1093/genetics/97.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley C. H., Voelker R. A., Brown A. J., Ohnishi S., Dickson B., Montgomery E. Null allele frequencies at allozyme loci in natural populations of Drosophila melanogaster. Genetics. 1981 Sep;99(1):151–156. doi: 10.1093/genetics/99.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa S. Clinical and biochemical studies on mutant red cell enzymes mainly associated with hemolytic anemia. Jinrui Idengaku Zasshi. 1980 Jun;25(2):83–92. doi: 10.1007/BF01873607. [DOI] [PubMed] [Google Scholar]
- Miwa S., Fujii H., Takegawa S., Nakatsuji T., Yamato K., Ishida Y., Ninomiya N. Seven pyruvate kinase variants characterized by the ICSH recommended methods. Br J Haematol. 1980 Aug;45(4):575–583. doi: 10.1111/j.1365-2141.1980.tb07181.x. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai T., Cockerham C. C. Spontaneous mutation rates at enzyme loci in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2514–2517. doi: 10.1073/pnas.74.6.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Hamilton H. B., Otake M., Goriki K., Kageoka T., Fujita M., Neriishi S., Asakawa J. Search for mutations affecting protein structure in children of atomic bomb survivors: preliminary report. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4221–4225. doi: 10.1073/pnas.77.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R. R., Langley C. H., Voelker R. A. Enzyme mutants induced by low-dose-rate gamma-irradiation in Drosophila: frequency and characterization. Environ Mutagen. 1980;2(2):167–177. doi: 10.1002/em.2860020209. [DOI] [PubMed] [Google Scholar]
- Schull W. J., Otake M., Neel J. V. Genetic effects of the atomic bombs: a reappraisal. Science. 1981 Sep 11;213(4513):1220–1227. doi: 10.1126/science.7268429. [DOI] [PubMed] [Google Scholar]
- Soares E. R. TEM-induced gene mutations at enzyme loci in the mouse. Environ Mutagen. 1979;1(1):19–25. doi: 10.1002/em.2860010108. [DOI] [PubMed] [Google Scholar]
- TAKAHARA S., HAMILTON H. B., NEEL J. V., KOBARA T. Y., OGURA Y., NISHIMURA E. T. Hypocatalasemia: a new genetic carrier state. J Clin Invest. 1960 Apr;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE W. N., TANAKA K. R., MIWA S. A specific erythrocyte glycolytic enzyme defect (pyruvate kinase) in three subjects with congenital non-spherocytic hemolytic anemia. Trans Assoc Am Physicians. 1961;74:100–110. [PubMed] [Google Scholar]
- Voelker R. A., Langley C. H., Brown A. J., Ohnishi S., Dickson B., Montgomery E., Smith S. C. Enzyme null alleles in natural populations of Drosophila melanogaster: Frequencies in a North Carolina population. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1091–1095. doi: 10.1073/pnas.77.2.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R. A., Schaffer H. E., Mukai T. Spontaneous Allozyme Mutations in DROSOPHILA MELANOGASTER: Rate of Occurrence and Nature of the Mutants. Genetics. 1980 Apr;94(4):961–968. doi: 10.1093/genetics/94.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]


