Abstract
When a previously common predator disappears owing to local extinction, the strong source of natural selection on prey to visually recognize that predator becomes relaxed. At present, we do not know the extent to which recognition of a specific predator is generalized to similar looking predators or how a specific predator-recognition cue, such as coat pattern, degrades under prolonged relaxed selection. Using predator models, we show that deer exhibit a more rapid and stronger antipredator response to their current predator, the puma, than to a leopard displaying primitive rosettes similar to a locally extinct predator, an early jaguar. Presentation of a novel tiger with a striped coat engendered an intermediate speed of predator recognition and strength of antipredator behaviour. Responses to the leopard model slightly exceeded responses to a non-threatening deer model, suggesting that thousands of years of relaxed selection have led to the loss of recognition of the spotted coat as a jaguar-recognition cue, and that the spotted coat has regained its ability to camouflage the felid form. Our results shed light on the evolutionary arms race between adoption of camouflage to facilitate hunting and the ability of prey to quickly recognize predators by their formerly camouflaging patterns.
Keywords: relaxed selection, predator recognition, camouflage, deer, puma, jaguar
1. Introduction
Ungulates are morphologically and behaviourally adapted to react quickly to the presence of predators and escape capture using effective flight strategies. While morphological adaptations for rapid, prolonged flight appear to be retained for thousands of generations despite the local extinction of most major predatory species (Byers 1999), olfactory and auditory recognition appear to disintegrate rapidly in the absence of predators (Blumstein 2002). Berger and colleagues found that populations of moose (Alces alces) that were lacking predators for as few as 50–130 years showed reduced responses to auditory and olfactory predator cues compared with predator-experienced moose (Berger et al. 2001). Similarly, predator-naive tammar wallabies (Macropus eugenii) and red-necked pademelons (Thylogale thetis) did not alter feeding behaviour in the presence of formerly relevant predator odours compared with control conditions (Blumstein et al. 2002). However, both studies suggested that these sensory abilities can be restored in as little as one generation, presumably by learning about predators. When presented with purely visual stimuli, some mammals show evolutionary persistence of recognition after an estimated 300 thousand years (kyr) of prolonged relaxed selection (Coss 1991, 1999), albeit with complete decay of predator recognition in 3–5 million years (Myr; Goldthwaite et al. 1990). However, there is evidence that tammar wallabies that have been isolated from predators for only 130 years showed a loss of visual predator recognition (Blumstein et al. 2004).
The multi-predator hypothesis (Blumstein et al. 2004; Blumstein 2006) predicts that antipredator behaviour towards a particular predator may be maintained if prey continue to encounter a similar predatory species. While there is evidence to support the multi-predator hypothesis (Blumstein & Daniel 2003; Blumstein et al. 2004), selection from specific predators can be asymmetrical in contexts in which one of the two historical predators disappears. For instance, wild-caught California ground squirrels (Spermophilus beecheyi) that recently colonized a rattlesnake-rare site did not differentiate the venomous rattlesnake (Crotalus oreganus) and non-venomous gopher snake (Pituophis melanoleucus), whereas ground squirrels whose ancestors had experienced more prolonged relaxed selection from rattlesnakes were more aggressive towards their extant gopher snake predator than to rattlesnakes (Coss et al. 1993). Our present research on Columbian black-tailed deer (Odocoileus hemionus columbianus) examines a similar phenomenon in which one of the two large, distinctively looking felid predators becomes extinct locally, yielding prolonged relaxed selection on recognition of the extinct predator in a time frame spanning hundreds of thousands of years.
In North America, the Late Hemphillian fossil record (Woodburne & Swisher 1995; Morgan & Lucas 2003; Barnosky & Shabel 2005) suggests a long history of felid–deer interactions and strong natural selection for rapid recognition of danger by deer. The first wave of immigration of Odocoileini cervids from Asia to North America via Beringia occurred during the Late Hemphillian about 5.5 Myr ago followed by the immigration of true Odocoileus during the Early Blancan (Woodburne & Swisher 1995; Morgan & Lucas 2003). These early North American immigrants would have encountered felid predators Megantereon and Homotherium during the Middle to Late Blancan (Bell et al. 2004). Odocoileus deer have been in contact with extant pumas (Puma concolor) almost continuously since the Middle Pleistocene (see figure in the electronic supplementary material; Stock 1918; Simpson 1941; Barnosky & Shabel 2005). However, a genetic study suggests that North American pumas became extinct during the end of the Pleistocene, an event followed by rapid recolonization by a small number of founders (Culver et al. 2000). If this extinction event did occur, then black-tailed deer might have experienced a brief period of relaxed selection from all large felid predators.
Ancestral jaguars (Panthera aff. Panthera gombaszoegensis; Panthera onca augusta) immigrated from Asia during the Early Pleistocene and co-occured with Odocoileus approximately 1.6 Myr ago in California (see figure in the electronic supplementary material; Savage 1951; Firby 1968; Sarna-Wojcicki 1976; Kurtén & Anderson 1980). Possible contact between jaguars and the ancestors of black-tailed deer might have occurred in Washington approximately 610 kyr ago and in Oregon during the Late Irvingtonian to Early Rancholabrean (Elftman 1931; Simpson 1941; Gustafson & Fry 1974), but sympatry this recent is ambiguous at best. The distribution of fossil sites exhibiting the large jaguar (P. onca augusta) indicates a progressive regression of the distribution of jaguars from the Northwestern USA in the Late Irvingtonian to the Southwestern USA in more recent times (for maps showing historical and modern distributions of jaguars in North America see Daggett & Henning (1974) and Seymour (1989)). Genetic analysis coupled with the fossil record suggests that the mule deer (Odocoileus hemionus) progenitor of black-tailed deer diverged from white-tailed deer (Odocoileus virginianus) approximately 2.8 Myr ago (Pitra et al. 2004), following which Odocoileus along the Northern and Southern California coast were isolated by the onset of drainage of the Sacramento River through the San Francisco Bay, approximately 725 kyr ago (Smith & Coss 1984). Such a barrier to gene flow led to the genetic divergence of mule deer (Odocoileus hemionus californicus), black-tailed deer (Odocoileus hemionus columbianus) and pumas along the California coast (Carr & Hughes 1993; Ernest et al. 2003) and the isolation of black-tailed deer in Northwestern California and jaguars in Southern California, where they were already rare in the Late Pleistocene (Jefferson 1983). Viewed together with the jaguar fossil record, the mule deer ancestors of black-tailed deer and black-tailed deer themselves were likely prey of early jaguars for possibly 1 Myr before gene flow along the California coast was disrupted.
Studies of the camouflaging properties of felid coats (Mottram 1915; Cott 1940; Godfrey et al. 1987; Ortolani 1999) suggest that vertical stripes should afford concealment in habitats where tall grass or reeds predominate. ‘Obliterative shading’ is produced by a darker dorsal and lighter ventral coat to diminish perception of the cylindrical form. However, the obliterative shading of jaguars and leopards is much less pronounced than that of tigers, but the rosettes of these cats might produce the functional equivalent of obliterative shading by a dorsal-to-ventral lightening of colour patches within the centre of these rosettes (Mottram 1915). Ironically, rather than acting as camouflage, the repetitive texture of the leopard's spotted coat is highly salient to a variety of prey, including cervids, suggesting that an evolutionary arms race (Dawkins & Krebs 1979) has unfolded, in which the presumed original camouflaging function of the spotted coat has now become a major leopard-recognition cue by prey. For example, presentations of realistic models of a spotted leopard generated rapid and vigorous alarm calling by wild deer, wild primates, and even captive-born female rhesus macaques (Macaca mulatta) and inexperienced urban bonnet macaques (Macaca radiata; Coss & Ramakrishnan 2000; Ramakrishnan & Coss 2000a,b; Hollis-Brown 2005). Further, bonnet macaques recognized leopards partially obscured by vegetation, predominantly by their spotted yellow coat (Coss et al. 2005). Together, these results suggest that rather than having camouflaging properties, the pattern regularity of the spotted yellow coat is highly conspicuous among species that have coevolved with leopards for thousands of generations. Therefore, a 1 Myr time frame of predation by jaguars on deer was probably sufficient for the evolution of jaguar-specific recognition in which the jaguar's coat pattern of flecks and rosettes would have become an important jaguar-recognition cue. An analogous perceptual capitalization on pattern regularity with putative concealing properties is apparent for snake recognition by ground squirrels in which snake scale patterns are readily detected (Coss 1991).
Although we are confident that coats of early jaguars were spotted, there is uncertainty about the coat patterns of two other large extinct felid predators, the sabre-toothed cat (Smilodon fatalis) and the North American lion (Panthera leo atrox), that appear with black-tailed deer in the same Late Pleistocene fossil assemblage (Stock 1918). While the coat pattern of Smilodon is unknowable unless future fossil DNA analyses reveals it, European cave lions (Panthera leo spelaea) are depicted in Upper Palaeolithic cave paintings and drawings as maneless and spotless (cf. Wildgen 2004; Yamaguchi et al. 2004).
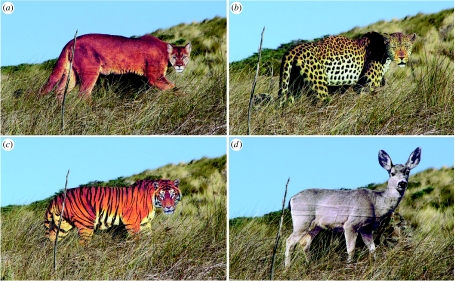
The difference in selective history of predation from large cats with a uniform coat pattern (current exposure) and a cat with a presumed coat pattern of flecks and rosettes (now absent), along with the presumption that prolonged evolutionary exposure to a given predator will select for rapid recognition of that predator, was used to develop the hypothesis that, owing to prolonged relaxed selection possibly spanning up to 700 kyr, black-tailed deer should show much weaker antipredator behaviour during exposure to a realistic looking model of a spotted cat than to a model of a puma. Similarly, if coat texture disrupts felid recognition as proposed for the vertical stripes of tigers (Mottram 1915; Godfrey et al. 1987), a completely novel tiger model, a species with no historical relevance and no opportunity for selection for rapid recognition, should elicit an even weaker antipredator response. To understand the roles of coat pattern and relaxation of selection from predators on visual predator recognition, we compared antipredator behaviours of adult black-tailed deer in Northern California, where the only large felid predator is the puma, during and after systematic exposure to life-sized, two-dimensional models (figure 1) of a puma, tiger and leopard (with a coat pattern of smaller, primitive rosettes as the reasonable coat texture of early jaguars; Werdelin & Olsson 1997). A model female mule deer (Odocoileus hemionus hemionus) was used as a non-threatening control. Since coat pattern is only one perceptual cue complementing the general felid form, we predicted that all the felid models would be more provocative than the mule deer control owing to the multi-million year history that deer have experienced predation by large felids.
Figure 1.
Models presented to black-tailed deer shown to scale: (a) puma, (b) leopard, (c) tiger and (d) mule deer.
2. Material and methods
(a) Study sites
All trials were performed on free-living adult deer at Point Reyes National Seashore (PRNS) in Marin County, California, and on the Bodega Peninsula (BP) in Sonoma County, California between June and September in 2004 and 2005. Vegetation ranged from grassland pasture (height less than 10 cm) to a mixture of scrub and tall grass and no tree cover, and terrain consisted of low rolling hills. Deer of PRNS and BP have been protected from human hunting for 30–40 years and the deer of BP have become highly habituated to humans. Confirmed puma sightings and kill carcasses are sporadic but not unusual at PRNS; there have been several reliable puma sightings in the last 10 years at BP (P. Conners 2006, personal communication). The size of BP, puma homerange size, the stealthy habits of pumas and the presence of pumas in surrounding areas suggest that pumas do occasionally visit BP. Similar to most studies on wild deer, individuals were neither marked nor identifiable by physical characteristics. However, we strengthened confidence that replication of focal sampling was rare by avoiding resampling of what appeared to be the same group within 1–2 weeks, by conducting trials in different locations, and by attempting to sample different focal individuals within the group if it was likely that the group had been studied previously.
(b) Models
Images for the models were electronically scanned from high-quality colour photographs in books and digitally tailored to (i) achieve the same body and head orientation (i.e. lateral view of the body, head turned to face the observer and body in a stalking position with scapula forming a hump behind the head), (ii) increase coat pattern regularity, (iii) remove shadows falling on the head and (iv) remove blemishes. The images were printed on heavyweight matte paper on a Designjet 800 poster printer, permanently mounted on foam board with a flexible horizontal hinge in the centre, spray-painted green/brown camouflage on the reverse side, and sprayed with matte finish polyurethane water-repellant. Owing to the difficult logistics of testing deer unfamiliar with the models as described in §2c, only one model of each species was constructed. To avoid differences in perceptual loading, all models have approximately the same total surface area and the felid models have similar body lengths (head to base of tail).
(c) Model exposure procedure
Animals in relatively open areas with little occlusive scrub were targeted for exposure. The location of the model relative to the deer had to fit several criteria. The model was positioned 15–70 m from the focal individual; a closer position may result in the deer being surprised by the sudden appearance of an object and deer would have had greater difficulty discerning the shape and pattern of the model from distances beyond this range. The location had to provide sufficient visual cover for the experimenter and the model, so that the deer would not naturally know what was behind the source of cover (e.g. hillside or bush). All trials were conducted so that the model was downwind of the deer group to avoid confounding results with odour cues. Finally, a position was selected that provided direct sunlight on the image of the model, to avoid a backlit silhouette that would make the image/coat pattern less visible. Using a slowly moving car as temporary cover, the experimenter, hiding under a green camouflage mesh sheet, covertly positioned himself with the model behind the protective cover so that the deer did not know he was there; if he was detected prematurely, the trial was aborted. All behaviour was videotaped by a second observer using a Panasonic model PV-DV601D mini digital video recorder from a greater distance shown not to influence deer activity in a previous study (Stankowich & Coss 2006). The experimenter erected the model into view and once the focal deer detected the model, it was held in view for 30 s before it was concealed again. The model was exposed up to four times per trial with approximately 1 min intervals between each exposure. Once the last exposure was completed, the experimenter laid on the ground silently under the green tarp, and video recording continued until the deer were out of visual contact or began feeding continuously for more than 2 min.
(d) Behaviour quantification
We recorded (i) the distance between the model and the focal individual for each exposure using a Bushnell Yardage Pro laser rangefinder accurate to the nearest 1 m, (ii) group size and composition and (iii) environmental factors (temperature, wind speed/direction and weather). Both the experimenter and video camera operator counted the number of snorts (audible expulsions of air through the nose) heard during the course of the trial. Since most snorts were not audible on the videotape, we only analysed the total number of snorts we heard while performing the trial. It was not possible to identify which individual emitted each snort nor was it possible to record the timing of each snort. The time of each foot-stamp performed by the focal deer was determined from the digital video. When deer were alarmed by the models, we often observed a behaviour which we called ‘alarm-walking’, where the deer walk more slowly and the limbs are raised in the air in an exaggerated fashion. This behaviour has been observed in the same predator–prey circumstances in mule deer (O. h. hemionus; V. Geist 2004, personal communication) but owing to the rarity of observing these encounters, the behaviour has never been described or quantified before in the literature. Alarm-walking is a qualitatively different style of walking than the normal deer gait (Stankowich & Coss, in preparation). The time and duration of each bout of alarm-walking by the focal deer was recorded from digital video. We recorded the time when the focal deer first detected the model when it was exposed, and foot-stamping and alarm-walking latency measurements were calculated relative to this time. Snorting and foot-stamping rates were calculated by dividing the total number of each behaviour by the duration of the trial, from the moment of first detection through 10 min past the end of the final model exposure.
(e) Analyses
Preliminary inspection of the data revealed that there were no differences in antipredator responses between the two sites, so that sites were not compared. The effect of the models on the frequency of deer snorting, foot-stamping and alarm-walking was examined using multinomial loglinear analysis with maximum-likelihood estimation and partitioned χ2-tests for planned specific model comparisons (Agresti 1990). Latencies to foot-stamp and alarm walk to each model type were compared with Kaplan–Meier survival analysis using the log-rank statistic (L) for planned pairwise model comparisons. Rates of snorting and foot-stamping behaviours were natural log transformed, grouped together and analysed using backward elimination MANCOVA (i.e. all factors were initially introduced into the model and the least significant factor was removed at each step until a final model was achieved with all factors significant at the 0.10 level; statistical significance was reached at α=0.05). Initial factors included in the analyses were model type, presence/absence of a fawn in the group, group size, initial distance between the model and the focal deer, and the interaction between the model and the fawn presence/absence. The difference in foot-stamping rates between the time periods when the exposures were occurring and the 10 min period after the final model exposure was analysed with the Wilcoxon signed-rank test. The difference in frequency of foot-stamps during the exposure period between when the model was exposed and the 30 s period after exposures was also analysed with the Wilcoxon signed-rank test. Post hoc multiple comparisons of behaviour rates were performed with Tamhane's correction when error variances were unequal (as determined by Levene's tests), and with Bonferroni's correction when error variances were equal. All analyses were performed with SPSS v. 10.0, and statistical significance for all tests was reached at α=0.05.
3. Results
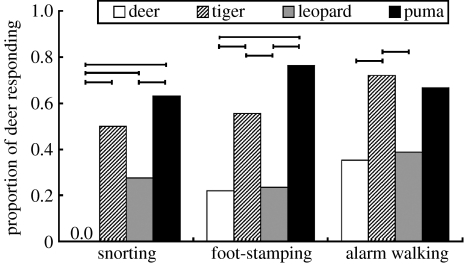
Responses to the deer model were minimal: there were no occurrences of snorting and only three occurrences of foot-stamping when exposed to the deer model. Both snorting and foot-stamping have been found to be indicators of recognition of danger and alarm in many ungulate species (Caro 2005). Similarly, a larger proportion of deer snorted, foot-stamped or alarm-walked in the presence of the predator models than with the non-threatening deer model (figure 2; loglinear χ2; snorting, χ32=23.840, p<0.001; foot-stamping, χ32=14.853, p=0.002; alarm-walking, χ32=7.758, p=0.051), indicating that the presentation protocol and two-dimensionality of the models probably did not affect the results, and any differences between the predator models is owing to their appearance and not other motion, auditory, olfactory or environmental cues. When compared individually, each predator model elicited snorting in a larger proportion of deer than the deer model (loglinear χ2; all p≤0.005), but only the tiger and the puma models elicited foot-stamping in a larger proportion of deer than the deer model (tiger, χ12=4.314, p=0.038; puma, χ12=10.872, p<0.001) and a larger proportion of deer only alarm-walked to the tiger more than to the deer model (χ12=4.918, p=0.027). The deer and leopard had nearly identical frequencies of foot-stamping and alarm-walking (p>0.500). A larger proportion of deer snorted or foot-stamped at the puma model than at the leopard (snorting, χ12=4.773, p=0.029; foot-stamping, χ12=10.034, p=0.002) and more deer foot-stamped or alarm-walked at the tiger model than at the leopard (foot-stamping, χ12=3.803, p=0.050; alarm-walking, χ12=4.134, p=0.042). The frequency of deer snorting or foot-stamping in the presence of the tiger was intermediate to that of the leopard and puma, but only the differences in foot-stamping and alarm-walking frequencies between the tiger and leopard were statistically significant. Therefore, all three behaviours were strongly associated with the presence of a predator and nearly absent when a non-threatening model was displayed, albeit the antipredator response to the leopard model was markedly less than both the tiger and puma models.
Figure 2.
Proportions of deer snorting, foot-stamping and alarm-walking. Ends of lines above the bars indicate statistically significant pairwise differences between models (p<0.05). Snorting, npuma=19, nleopard=ntiger=ndeer=18; foot-stamping, ntiger=ndeer=18, nleopard=npuma=17; alarm-walking, ndeer=17, ntiger=nleopard=npuma=18.
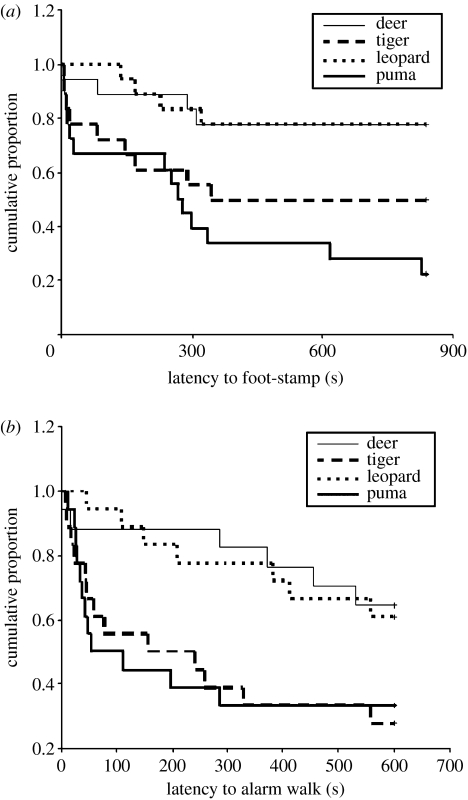
To measure the reaction time of predator recognition, we examined the latency to foot-stamp and alarm walk from the time of first detection of the model. Latency to foot-stamp was much faster in response to the tiger and puma models than to the leopard and deer models (figure 3a). Deer foot-stamped to the puma model significantly sooner than to both the deer (survival analysis, L=10.83, p=0.001) and leopard models (L=10.97, p=0.001). There was a trend for faster reaction to the tiger model than to both the deer (L=3.44, p=0.064) and leopard models (L=3.14, p=0.076), but neither pairwise comparison showed reliable differences. There were no statistically significant differences in reaction time between the leopard and deer models (L=0.00, p=0.966) and the puma and tiger models (L=1.90, p=0.168). The same trends were apparent for alarm-walking reaction times: latencies to alarm walk were much faster in response to the tiger and puma models than to the leopard and deer models (figure 3b). Deer alarm-walked to the puma model significantly sooner than to both the deer (survival analysis, L=4.56, p=0.033) and leopard models (L=4.99, p=0.025). Similarly, the latencies to alarm walk were also much shorter to the tiger model than to the deer (L=5.76, p=0.016) and leopard models (L=5.58, p=0.018). There were no reliable differences between the puma and tiger models (L=0.00, p=0.976) and between the deer and leopard models (L=0.04, p=0.841).
Figure 3.
Plots of Kaplan–Meier survivorship functions of latencies to (a) foot-stamp and (b) alarm walk to each model. (a) Pairwise comparisons indicate statistically significant differences (p<0.05) between the puma and deer models and between the puma and leopard models (npuma=19, nleopard=ntiger=ndeer=18). (b) Pairwise comparisons indicate that both the puma and tiger models are statistically significantly different (p<0.05) from both the deer and leopard models (ndeer=17, ntiger=nleopard=npuma=18).
The size of the deer group had no effect on antipredator behaviour rates (MANCOVA, F2,62=0.311, p=0.734), and, in the range of values tested, the initial distance between the deer and the model did not influence behaviour (MANCOVA, F2,66=0.556, p=0.576). Deer foot-stamped at a higher rate when there were no fawns present (0.717±0.237 (mean±s.e.m.), n=25) than when there was a fawn present in the group (0.223±0.084, n=48; MANCOVA, between-subjects F1,68=5.366, p=0.024).
Models differed significantly in their effect on overall response strength (MANCOVA, F6,136=3.575, p=0.003) and individually on snorting rate (between-subjects F3,68=4.603, p=0.005) and foot-stamping rate (between-subjects F3,68=4.066, p=0.010). Deer snorted at a higher rate to the puma and tiger than to the deer (puma–deer, p=0.012; tiger–deer, p=0.046). The tiger and puma models elicited similar snort rates, and the leopard did not differ from any of the other models (all p>0.05). Deer foot-stamped at a higher rate when exposed to the puma than when exposed to the deer (p=0.030) or leopard models (p=0.049). There were no other pairwise differences in foot-stamping rates among the models (all p>0.05). Deer foot-stamped more when the model was actively appearing and disappearing than after the model disappeared from view for the last time (Wilcoxon, Z=−3.175, p=0.001). However, during the period of the trial with active exposure and concealment (i.e. up to 30 s past the end of the final exposure), there was no difference in the frequency of foot-stamps when the model was exposed and when it was concealed (Wilcoxon, Z=−0.420, p=0.675).
4. Discussion
Both snorting and foot-stamping have been found to be indicators of recognition of danger and alarm in many ungulate species (Caro 2005). Similarly, both snorting and foot-stamping were strongly associated with the presence of a predator and absent when a non-threatening model was displayed, albeit the antipredator response to the leopard model was markedly less than both the tiger and puma models. Additionally, responses to the deer were much slower and weaker compared with the tiger and puma models and, to some extent, the leopard model. These results indicate that the presentation protocol and two-dimensionality of the models probably had little effect on the results and any differences between the predator models are owing to their appearance and not other motion, auditory, olfactory or environmental cues.
In nearly all analyses, the puma showed the strongest effect on deer responses. With the exception of the frequency of foot-stamping and alarm-walking, the tiger was intermediate to, but not reliably different from, the leopard and puma models. Deer were most fearful of the puma, and the puma elicited the strongest overall response of any of the predator models, suggesting that the individuals tested either occasionally came into contact with pumas directly (we know this to be the case with PRNS) or have retained rapid recognition of pumas via vertical inheritance of puma-recognition ability from recent ancestors that did encounter pumas directly. While mean strengths of behaviour towards the tiger model were consistently equal to or below those for the puma model, we found no reliable differences between responses to the tiger and puma models in any statistical comparison. The fact that there was no reduction in speed and strength of recognition of the tiger model, despite our presumption that these deer have not had any historical experience with a vertically striped predator, suggests that the tiger's vertical stripes did not sufficiently disrupt its general felid appearance and that deer were able to generalize the more familiar threatening puma configuration with a uniform coat to the novel striped cat. Our finding that the tiger was conspicuous to black-tailed deer in backgrounds with fine grass does not preclude the argument that tiger stripes evolved as camouflage with background-matching properties in South Asian habitats with much thicker grass and reeds (Mottram 1915; Godfrey et al. 1987).
Our results suggest that threat recognition occurs much more rapidly and is strongest for the puma and tiger models compared with the leopard model. Given current evidence for rapid recognition of and strong responses to spotted felids by mammalian prey (Coss & Ramakrishnan 2000; Ramakrishnan & Coss 2000b; Coss et al. 2005), we would expect O. hemionus to have had similarly refined recognition capabilities of spotted felids when jaguars were active predators of deer historically throughout North America. An alternative explanation to the re-emergence of spots as camouflage is that most or all deer perceived the spotted pattern of the leopard model, but that the dangerous meaning of spots had disintegrated under prolonged relaxed selection. However, if spots were indeed perceived as neutral and not as obliterative patterns, then recognition of the general felid form should have promoted much stronger responses. Moreover, the strong responses to the tiger model support the argument that the general felid form was still perceived as dangerous in the grassy background in which it was presented. By making this point, it becomes more convincing that spots have probably re-emerged as a camouflaging pattern following relaxed selection. Nevertheless, spots might not have acted as felid camouflage for all the deer. It must be noted that the snorting measure clearly indicated that some deer still perceived the leopard as dangerous, which suggests that (i) the spotted coat retained its dangerous meaning, (ii) the spotted coat did not obliterate the leopard's felid form completely in some backgrounds or (iii) some deer were more reactive or sensitive to novelty than others (Stankowich & Coss 2006). That deer did treat the tiger as dangerous (presumably because they can see a large cat) suggests that spots help the leopard blend into the background environment effectively. From this perspective, the spotted coat appears to have regained its assumed earlier camouflaging properties by disrupting the general felid configuration (Mottram 1915), resulting in a reduction in speed and strength of felid recognition. As such, there appears to be two competing perceptual processes at work in this system: (i) relaxation of selection leading to the loss of recognition of a formerly salient coat pattern as characterizing danger and (ii) the re-emergence of the edge-disruptive, blending properties of a spotted, camouflage pattern (Mottram 1915).
Effects of other factors on the performance of antipredator behaviours were minimal. The lack of effects of group size and initial model distance on snorting and foot-stamping rates suggests that antipredator behaviour is not assuaged in larger groups (cf. Blumstein et al. 2004) and that as long as detection/recognition occurs, longer distances to the predator do not influence the strength of the antipredator response. The presence of fawns in the group reliably decreased the rate of foot-stamping (n.b. additionally, fawns may have remained hidden in nearby vegetation throughout the trial and while post-trial measurements were taken). If foot-stamping functions as a conspecific warning signal (Caro et al. 2004), we would expect increased foot-stamping when fawns were present so as to warn them of the potential danger or to potentially sensitize them to the threat (e.g. Stankowich & Sherman 2002). This evidence appears contradictory to previous results (Caro et al. 2004) showing that foot-stamping occurs more often in species living in intermediate and large groups. We suggest that foot-stamping may function as a pursuit deterrent by drawing the attention of the predator and communicating that it has been spotted; deer may be less likely to foot-stamp to draw attention from a predator when fawns, which are less able to flee effectively, are present.
In light of previous findings on the role of conspicuous spots as salient rather than camouflaging patterns (Mottram 1915; Coss & Ramakrishnan 2000; Ramakrishnan & Coss 2000a,b; Coss et al. 2005; Hollis-Brown 2005), our results suggest that the spotted felid coat evolved initially to disrupt recognition of the felid form. As prey experienced a consistent source of natural selection when they failed to recognize spotted felid predators, adaptive changes in felid-recognition systems began to capitalize on the regularity of the spotted coat as a conspicuous predator-recognition cue. The apparent loss of recognition of the spotted coat as dangerous and the re-emergence of spots as an effective camouflage suggest that visual predator recognition is evolutionarily labile and that an evolutionary arms race exists between predator camouflage and prey recognition of predators. While the multi-predator hypothesis (Blumstein 2006) predicted that deer should retain recognition of the major felid form because pumas remain as a source of predation, we see that unique camouflaging patterns may limit an animal's ability to generalize current predators to those of the past. As such, we recommend that future research should focus on the dynamics between more recent relaxed selection and predator camouflage strategies and should attempt to use more complex predator models to learn what specific features of predators disrupt their recognition by prey.
Acknowledgments
This research was supported by grants from the University of California, Davis and the Bodega Marine Laboratory (BML). We thank E. Arnett, S. Mader, B. Main and several others for their assistance during data collection; M. Jaquez, R. Hultgren, B. Klinger, K. Luther, H. Nguyen and R. Nguyen for their assistance with videotape analyses; Peter Conners (former BML manager) for his information on puma presence on BP, and S. Lingle and T. Caro for their theoretical input during the planning stages. We thank D. Owings, A. Sih and two anonymous reviewers for their comments on previous drafts of this manuscript. This work was conducted under research permits from the California Departments of Fish and Game (803043-05) and Parks and Recreation, and the National Park Service (03-0024).
Supplementary Material
Horizontal dashed lines indicate transitions in North American land mammal ages. Vertical coloured lines characterize possible progenitors of later species.
References
- Agresti A. Wiley series in probability and mathematical statistics. Applied probability and statistics. Wiley; New York, NY: 1990. Categorical data analysis. [Google Scholar]
- Barnosky A.D, Shabel A.B. Comparison of mammalian species richness and community structure in historic and mid-Pleistocene times in the Colorado Rocky Mountains. Proc. Calif. Acad. Sci. 2005;56:50–61. [Google Scholar]
- Bell C.J, Lundelius E.L, Barnosky A.D, Graham R.W, Lindsay E.H, Ruez K.R, Jr, Semken H.A, Jr, Webb S.D, Zakrzewski R.J. The Blancan, Irvingtonian, and Rancholabrean mammal ages. In: Woodburne M.O, editor. Late Cretaceous and Cenozoic mammals of North America: biostratigraphy and geochronology. Columbia University Press; New York, NY: 2004. pp. 232–314. [Google Scholar]
- Berger J, Swenson J.E, Persson I.L. Recolonizing carnivores and naive prey: conservation lessons from Pleistocene extinctions. Science. 2001;291:1036–1039. doi: 10.1126/science.1056466. doi:10.1126/science.1056466 [DOI] [PubMed] [Google Scholar]
- Blumstein D.T. Moving to suburbia: ontogenetic and evolutionary consequences of life on predator-free islands. J. Biogeogr. 2002;29:685–692. doi:10.1046/j.1365-2699.2002.00717.x [Google Scholar]
- Blumstein D.T. The multipredator hypothesis and the evolutionary persistence of antipredator behavior. Ethology. 2006;112:209–217. doi:10.1111/j.1439-0310.2006.01209.x [Google Scholar]
- Blumstein D.T, Daniel J.C. Foraging behavior of three Tasmanian macropodid marsupials in response to present and historical predation threat. Ecography. 2003;26:585–594. doi:10.1034/j.1600-0587.2003.03516.x [Google Scholar]
- Blumstein D.T, Mari M, Daniel J.C, Ardron J.G, Griffin A.S, Evans C.S. Olfactory predator recognition: wallabies may have to learn to be wary. Anim. Conserv. 2002;5:87–93. doi:10.1017/S1367943002002123 [Google Scholar]
- Blumstein D.T, Daniel J.C, Springett B.P. A test of the multi-predator hypothesis: rapid loss of antipredator behavior after 130 years of isolation. Ethology. 2004;110:919–934. doi:10.1111/j.1439-0310.2004.01033.x [Google Scholar]
- Byers J.A. University of Chicago Press; Chicago, IL: 1999. American pronghorn: social adaptations and the ghosts of predators past. [Google Scholar]
- Caro T.M. University of Chicago Press; Chicago, IL: 2005. Antipredator defenses in birds and mammals. [Google Scholar]
- Caro T.M, Graham C.M, Stoner C.J, Vargas J.K. Adaptive significance of antipredator behaviour in artiodactyls. Anim. Behav. 2004;67:205–228. doi:10.1016/j.anbehav.2002.12.007 [Google Scholar]
- Carr S.M, Hughes G.A. Direction of introgressive hybridization between species of North American deer (Odocoileus) as inferred from mitochondrial cytochrome-b sequences. J. Mammal. 1993;74:331–342. doi:10.2307/1382388 [Google Scholar]
- Coss R.G. Context and animal behavior III: the relationship between early development and evolutionary persistence of ground squirrel antisnake behavior. Ecol. Psychol. 1991;3:277–315. doi:10.1207/s15326969eco0304_1 [Google Scholar]
- Coss R.G. Effects of relaxed selection on the evolution of behavior. In: Foster S.A, Endler J.A, editors. Geographic variation in behavior: perspectives on evolutionary mechanisms. Oxford University Press; New York, NY: 1999. pp. 180–208. [Google Scholar]
- Coss R.G, Ramakrishnan U. Perceptual aspects of leopard recognition by wild bonnet macaques (Macaca radiata) Behaviour. 2000;137:315–335. doi:10.1163/156853900502105 [Google Scholar]
- Coss R.G, Guse K.L, Poran N.S, Smith D.G. Development of antisnake defenses in California ground squirrels (Spermophilus beecheyi): II. Microevolutionary effects of relaxed selection from rattlesnakes. Behaviour. 1993;124:137–164. [Google Scholar]
- Coss R.G, Ramakrishnan U, Schank J. Recognition of partially concealed leopards by wild bonnet macaques (Macaca radiata)—the role of the spotted coat. Behav. Process. 2005;68:145–163. doi: 10.1016/j.beproc.2004.12.004. doi:10.1016/j.beproc.2004.12.004 [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen & Co; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Culver M, Johnson W.E, Pecon-Slattery J, O'Brien S.J. Genomic ancestry of the American puma (Puma concolor) J. Hered. 2000;91:186–197. doi: 10.1093/jhered/91.3.186. doi:10.1093/jhered/91.3.186 [DOI] [PubMed] [Google Scholar]
- Daggett P.M, Henning D.R. The jaguar in North America. Am. Antiq. 1974;39:465–469. doi:10.2307/279437 [Google Scholar]
- Dawkins R, Krebs J.R. Arms races between and within species. Proc. R. Soc. B. 1979;205:489–511. doi: 10.1098/rspb.1979.0081. [DOI] [PubMed] [Google Scholar]
- Elftman H.O. Pleistocene mammals of Fossil Lake, Oregon. Am. Mus. Novit. 1931;481:1–21. [Google Scholar]
- Ernest H.B, Boyce W.M, Bleich V.C, May B, Stiver S.J, Torres S.G. Genetic structure of mountain lion (Puma concolor) populations in California. Conserv. Genet. 2003;4:353–366. doi: 10.1023/A:1024069014911. doi:10.1023/A:1024069014911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firby, J. B. 1968 Revision of the middle Pleistocene Irvington fauna of California. M. A. in Paleontology, University of California.
- Godfrey D, Lythgoe J.N, Rumball D.A. Zebra stripes and tiger stripes—the spatial frequency distribution of the pattern compared to that of the background is significant in display and crypsis. Biol. J. Linn. Soc. 1987;32:427–433. [Google Scholar]
- Goldthwaite R.O, Coss R.G, Owings D.H. Evolutionary dissipation of an antisnake system: differential behavior by California and Arctic ground squirrels in above- and below-ground contexts. Behaviour. 1990;112:246–269. [Google Scholar]
- Gustafson E.P, Fry W.E. Cervids from Pliocene and Pleistocene of central Washington. J. Paleontol. 1974;48:375–386. [Google Scholar]
- Hollis-Brown, L. A. 2005 Individual variation in the antipredator behavior of captive rhesus monkeys (Macaca mulatta). Ph.D thesis, University of California, Davis, CA.
- Jefferson G.T. First record of jaguar Panthera onca—new record from the late Pleistocene of California USA. Bull. South. Calif. Acad. Sci. 1983;82:95–98. [Google Scholar]
- Kurtén B, Anderson E. Columbia University Press; New York, NY: 1980. Pleistocene mammals of North America. [Google Scholar]
- Morgan G.S, Lucas S.G. Mammalian biochronology of Blancan and Irvingtonian (Pliocene and early Pleistocene) faunas from New Mexico. Bull. Am. Mus. Nat. Hist. 2003;279:269–320. doi:10.1206/0003-0090(2003)279<0269:C>2.0.CO;2 [Google Scholar]
- Mottram J.C. Some observations on pattern-blending with reference to obliterative shading and concealment of outline. Proc. Zool. Soc. Lond. 1915:679–692. [Google Scholar]
- Ortolani A. Spots, stripes, tail tips and dark eyes: predicting the function of carnivore colour patterns using the comparative method. Biol. J. Linn. Soc. 1999;67:433–476. doi:10.1006/bijl.1998.0299 [Google Scholar]
- Pitra C, Fickel J, Meijaard E, Groves P.C. Evolution and phylogeny of old world deer. Mol. Phylogenet. Evol. 2004;33:880–895. doi: 10.1016/j.ympev.2004.07.013. doi:10.1016/j.ympev.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Coss R.G. Age differences in the responses to adult and juvenile alarm calls by bonnet macaques (Macaca radiata) Ethology. 2000a;106:131–144. doi:10.1046/j.1439-0310.2000.00501.x [Google Scholar]
- Ramakrishnan U, Coss R.G. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata) J. Comp. Psychol. 2000;114:3–12. doi: 10.1037/0735-7036.114.1.3. doi:10.1037/0735-7036.114.1.3 [DOI] [PubMed] [Google Scholar]
- Sarna-Wojcicki A.M. Correlation of late Cenozoic tuffs in the central coast ranges of California by means of trace- and minor-element chemistry. USGS Prof. Paper. 1976;972:1–30. [Google Scholar]
- Savage D.E. Late Cenozoic vertebrates of the San Francisco Bay region. Univ. Calif. Pub. Bull. Dept. Geol. Sci. 1951;28:215–314. [Google Scholar]
- Seymour K.L. Panthera onca. Mamm. Species. 1989;340:1–9. [Google Scholar]
- Simpson G.G. Large Pleistocene felines of North America. Am. Mus. Novit. 1941;1136:1–27. [Google Scholar]
- Smith D.G, Coss R.G. Calibrating the molecular clock—estimates of ground squirrel divergence made using fossil and geological time markers. Mol. Biol. Evol. 1984;1:249–259. doi: 10.1093/oxfordjournals.molbev.a040316. [DOI] [PubMed] [Google Scholar]
- Stankowich, T. & Coss, R. G. In preparation. The alarm walk of black-tailed deer:characterization of a new display.
- Stankowich T, Coss R.G. Effects of predator behavior and proximity on risk assessment by Columbian black-tailed deer. Behav. Ecol. 2006;17:246–254. doi:10.1093/beheco/arj020 [Google Scholar]
- Stankowich T, Sherman P.W. Pup shoving by adult naked mole-rats. Ethology. 2002;108:975–992. doi:10.1046/j.1439-0310.2002.00830.x [Google Scholar]
- Stock D. The Pleistocene fauna from Hawver cave. Univ. Calif. Pub. Bull. Dept. Geol. 1918;10:461–515. [Google Scholar]
- Werdelin L, Olsson L. How the leopard got its spots: a phylogenetic view of the evolution of felid coat patterns. Biol. J. Linn. Soc. 1997;62:383–400. doi:10.1006/bijl.1997.9999 [Google Scholar]
- Wildgen W. The Paleolithic origins of art, its dynamic and topological aspects, and the transition to writing. In: Marcel B, van Heusden B, Wildgen W, editors. Semiotic evolution and the dynamics of culture. Bern, Switzerland; Peter Lang: 2004. pp. 117–153. [Google Scholar]
- Woodburne M.O, Swisher C.C., III . Land mammal high-resolution geochronology, intercontinental overland dispersals, sea level, climate, and vicariance. In: Berggren W.A, Kent D.V, Aubry M.-P, Hardenbol J, editors. Geochronology, time scales, and global stratigraphic correlation. Special publication. vol. 54. Society for Economic Paleontology and Mineralogy; Tulsa, OK: 1995. pp. 335–364. [Google Scholar]
- Yamaguchi N, Cooper A, Werdelin L, Macdonald D.W. Evolution of the mane and group-living in the lion (Panthera leo): a review. J. Zool., Lond. 2004;263:329–342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Horizontal dashed lines indicate transitions in North American land mammal ages. Vertical coloured lines characterize possible progenitors of later species.