Abstract
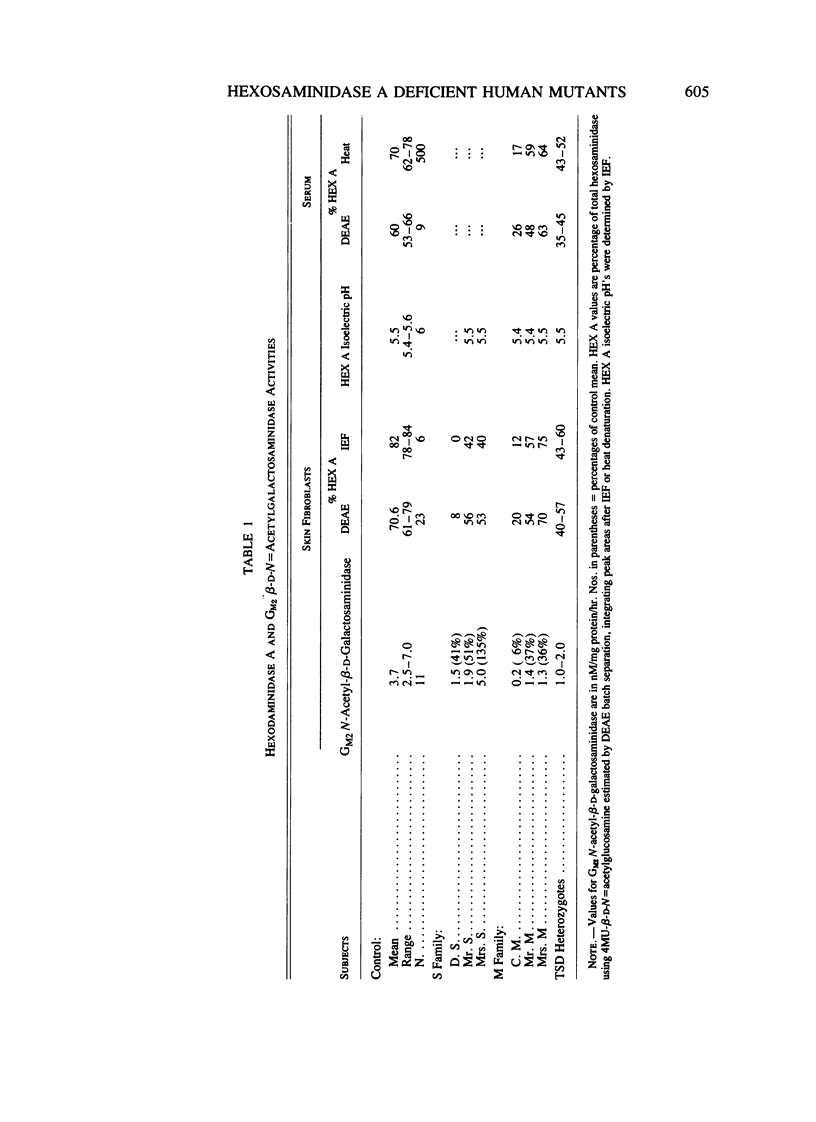
Two families with unusual hexosaminidase A (HEX A) mutations are described. In one, the proband had the Tay-Sachs disease phenotype with considerable HEX A activity. In the second, the proband was phenotypically normal with absent HEX A activity. Activities using ganglioside GM2 as substrate demonstrate markedly reduced activities in the first case and half-normal activities in the second. Pedigree analyses indicate the presence of two different mutations. In the first, the proband appears to be an allelic compound HEX A 2-4 where mutation HEX A 4 leads to a diminution of HEX A activity against GM2 but not for the synthetic substrate, 4MU-beta-D-N-acetyl-glucosaminide, with HEX A 2 being the Tay-Sachs disease (or similar) mutation. In the second family, the proband is an allelic compound HEX A 2-5 where mutation HEX A 5 leads to a diminution of HEX A activity against the synthetic substrate, 4MU-beta-D-N-acetyl-glucosaminide, but not for GM2. The presence of either mutation will lead to false-negative (HEX A 4) or false-positive (HEX A 5) assignments of heterozygosity or homozygosity for GM2 gangliosidosis when synthetic substrates are employed. In both families, DM2 N-acetyl-beta-D-galactosaminidase activity in fibroblasts was an accurate determinant of phenotype.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach G., Suzuki K. Heterogeneity of human hepatic H-acetyl-beta-D-hexosaminidose. A activity toward natural glycosphingolipid substrates. J Biol Chem. 1975 Feb 25;250(4):1328–1332. [PubMed] [Google Scholar]
- Dance N., Price R. G., Robinson D. Differential assay of human hexosaminidases A and B. Biochim Biophys Acta. 1970 Dec 29;222(3):662–664. doi: 10.1016/0304-4165(70)90193-5. [DOI] [PubMed] [Google Scholar]
- Dreyfus J. C., Poenaru L., Svennerholm L. Absence of hexosaminidase A and B in a normal adult. N Engl J Med. 1975 Jan 9;292(2):61–63. doi: 10.1056/NEJM197501092920201. [DOI] [PubMed] [Google Scholar]
- Geiger B., Arnon R. Chemical characterization and subunit structure of human N-acetylhexosaminidases A and B. Biochemistry. 1976 Aug 10;15(16):3484–3493. doi: 10.1021/bi00661a014. [DOI] [PubMed] [Google Scholar]
- Kelly T. E., Reynolds L. W., O'Brien J. S. Segregation within a family of two mutant alleles for hexosaminidase A. Clin Genet. 1976 May;9(5):540–543. doi: 10.1111/j.1399-0004.1976.tb01609.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leroy J. G., Ho M. W., MacBrinn M. C., Zielke K., Jacob J., O'Brien J. S. I-cell disease: biochemical studies. Pediatr Res. 1972 Oct;6(10):752–757. doi: 10.1203/00006450-197210000-00002. [DOI] [PubMed] [Google Scholar]
- Li Y. T., Mazzotta M. Y., Wan C. C., Orth R., Li S. C. Hydrolysis of Tay-Sachs ganglioside by beta-hexosaminidase A of human liver and urine. J Biol Chem. 1973 Nov 10;248(21):7512–7515. [PubMed] [Google Scholar]
- Navon R., Padeh B., Adam A. Apparent deficiency of hexosaminidase A in healthy members of a family with Tay-Sachs disease. Am J Hum Genet. 1973 May;25(3):287–293. [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. S. Molecular genetics of GM1 beta-galactosidase. Clin Genet. 1975 Nov;8(5):303–313. [PubMed] [Google Scholar]
- O'Brien J. S., Norden G. W., Miller A. L., Frost R. G., Kelly T. E. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase and asialo GM2 (GA2) N-acetyl-beta-D-galactosaminidase; studies in human skin fibroblasts. Clin Genet. 1977 Mar;11(3):171–183. doi: 10.1111/j.1399-0004.1977.tb01296.x. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Okada S., Chen A., Fillerup D. L. Tay-sachs disease. Detection of heterozygotes and homozygotes by serum hexosaminidase assay. N Engl J Med. 1970 Jul 2;283(1):15–20. doi: 10.1056/NEJM197007022830104. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S. Suggestions for a nomenclature for the GM2 gangliosidoses making certain (possibly unwarrantable) assumptions. Am J Hum Genet. 1978 Nov;30(6):672–675. [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Wässle W. Anreicherung und Charakterisierung zweier Formen der menschlichen N-acetyl- -D-hexosaminidase. Hoppe Seylers Z Physiol Chem. 1971 Aug;352(8):1119–1133. [PubMed] [Google Scholar]
- Seyama Y., Yamakawa T. Multiple components of beta-N-acetylhexosaminidase from equine kidney. Their action on glycolipids and allied oligosaccharides. J Biochem. 1974 Mar;75(3):495–507. doi: 10.1093/oxfordjournals.jbchem.a130418. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Navon R., Padeh B. Ganglioside catabolism in hexosaminidase A-deficient adults. Nature. 1974 Nov 15;252(5480):254–255. doi: 10.1038/252254a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. N., Stoolmiller A. C., Matalon R., Dorfman A. N-acetyl-beta-hexosaminidase: role in the degradation of glycosaminoglycans. Science. 1973 Aug 31;181(4102):866–867. doi: 10.1126/science.181.4102.866. [DOI] [PubMed] [Google Scholar]
- Tomasi L. G., Fukushima D. K., Kolodny E. H. Steroid hexosaminidase activity in Tay-Sachs and Sandhoff-Jatzkewitz diseases. Neurology. 1974 Dec;24(12):1158–1165. doi: 10.1212/wnl.24.12.1158. [DOI] [PubMed] [Google Scholar]
- Vidgoff J., Buist N. R., O'Brien J. S. Absence of -N-acetyl-D-hexosaminidase A activity in a healthy woman. Am J Hum Genet. 1973 Jul;25(4):372–381. [PMC free article] [PubMed] [Google Scholar]
- Wenger D. A., Okada S., O'Brien J. S. Studies on the substrate specificity of hexosaminidase A and B from liver. Arch Biochem Biophys. 1972 Nov;153(1):116–129. doi: 10.1016/0003-9861(72)90427-4. [DOI] [PubMed] [Google Scholar]


