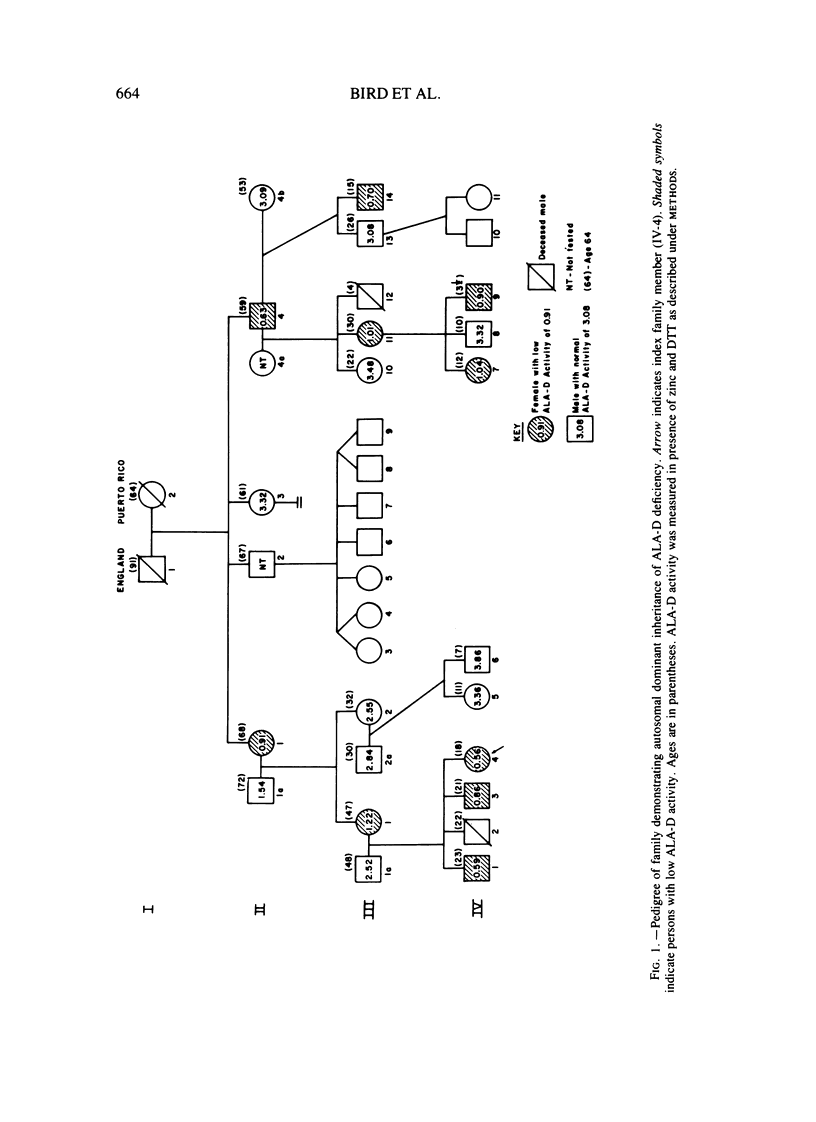
Abstract
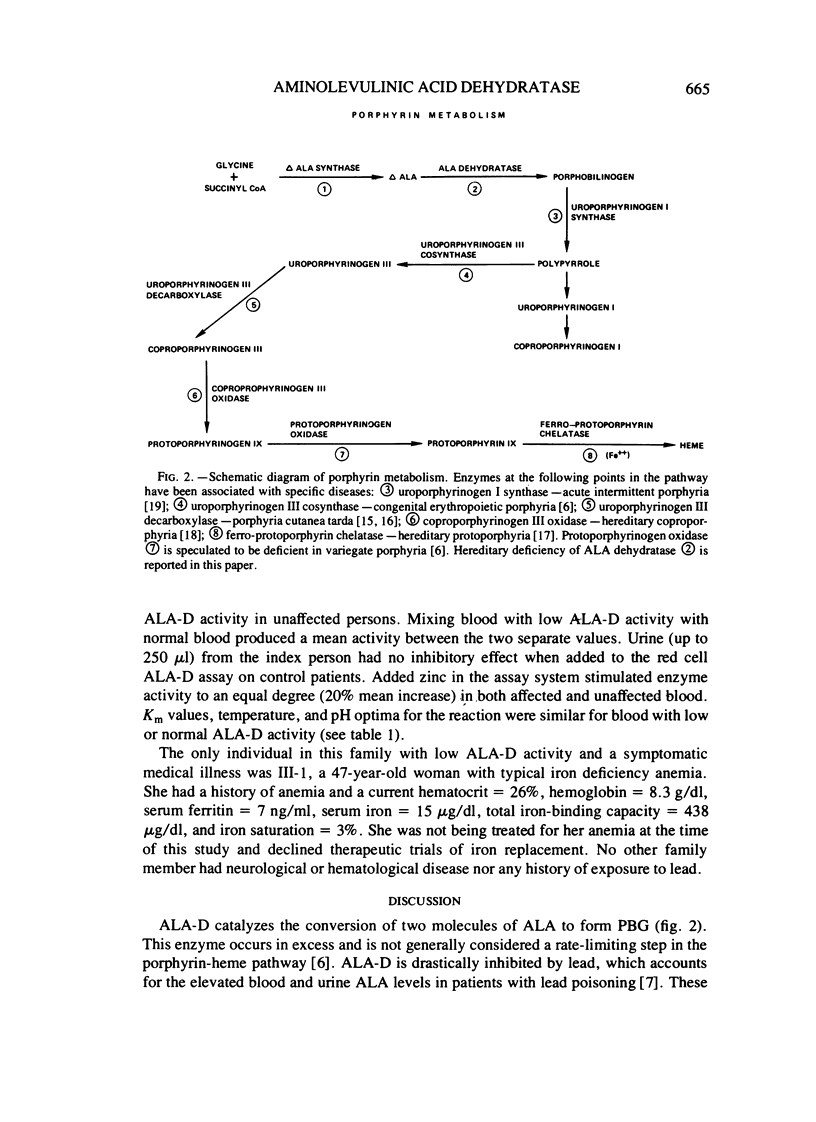
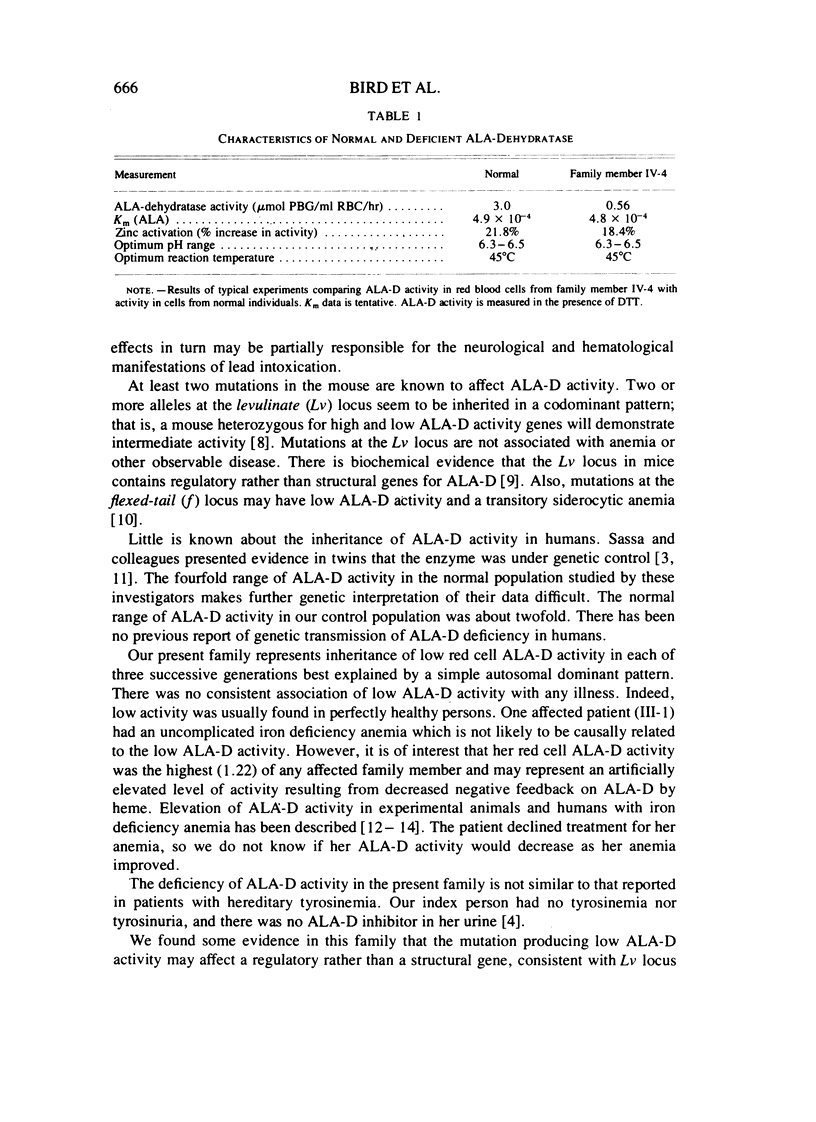
Delta-aminolevulinic acid dehydratase (ALA-D) is the second enzyme in the porphyrin-heme pathway and converts delta-aminolevulinc acid (ALA) to porphobilinogen (PBG). A family is reported with an inherited deficiency of red cell ALA-D activity occurring over three generations in an autosomal dominant pattern. Intial experiments support the hypothesis that the mutation in this family may affect a regulatory gene, but enzyme purification and further study are required. Although no clinical manifestations of deficient ALA-D activity have been found in affected persons, families such as this may be at increased risk for the serious consequences of lead poisoning, which produces marked inhibition of ALA-D activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie M. J., Thompson G. G., Moore M. R., Beattie A. D., Goldberg A. Hereditary coproporphyria. Demonstration of the abnormalities in haem biosynthesis in peripheral blood. Q J Med. 1977 Apr;46(182):229–241. [PubMed] [Google Scholar]
- Burch H. B., Siegel A. L. Improved method for measurement of delta-aminolevulinic acid dehydratase activity of human erythrocytes. Clin Chem. 1971 Oct;17(10):1038–1041. [PubMed] [Google Scholar]
- Chalevelakis G., Lyberatos C., Manopoulos D., Pyrovolakis J., Gardikas C. Erythrocyte delta-aminolaevulinic acid dehydratase, urinary porphyrins and porphyrin precursors in iron deficiency anaemia. Acta Haematol. 1977;57(5):305–309. doi: 10.1159/000207895. [DOI] [PubMed] [Google Scholar]
- Doyle D., Schimke R. T. The genetic and developmental regulation of hepatic delta-aminolevulinate dehydratase in mice. J Biol Chem. 1969 Oct 25;244(20):5449–5459. [PubMed] [Google Scholar]
- Elder G. H., Lee G. B., Tovey J. A. Decreased activity of hepatic uroporphyrinogen decarboxylase in sporadic porphyria cutanea tarda. N Engl J Med. 1978 Aug 10;299(6):274–278. doi: 10.1056/NEJM197808102990603. [DOI] [PubMed] [Google Scholar]
- Finelli V. N., Murthy L., Peirano W. B., Petering H. G. Delta-aminolevulinate dehydratase, a zinc dependent enzyme. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1418–1424. doi: 10.1016/0006-291x(74)90356-8. [DOI] [PubMed] [Google Scholar]
- Haeger-Aronsen B., Abdulla M., Fristedt B. I. Effect of lead on -aminolevulinic acid dehydrase activity in red blood cells. Arch Environ Health. 1971 Dec;23(6):440–445. doi: 10.1080/00039896.1971.10666033. [DOI] [PubMed] [Google Scholar]
- Hutton J. J., Coleman D. L. Linkage analyses using biochemical variants in mice. II. Levulinate dehydratase and autosomal glucose 6-phosphate dehydrogenase. Biochem Genet. 1969 Oct;3(5):517–523. doi: 10.1007/BF00485612. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé R. F., Finch C. A. Effects of iron status on delta-aminolevulinic acid dehydratase activity. Biochem Med. 1977 Dec;18(3):323–329. doi: 10.1016/0006-2944(77)90067-9. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Lindstedt S., Steen G. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4641–4645. doi: 10.1073/pnas.74.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis F. L., Russell E. S. Delta-aminolevulinate dehydratase activity in mice with hereditary anemia. Science. 1965 Oct 22;150(3695):496–497. doi: 10.1126/science.150.3695.496. [DOI] [PubMed] [Google Scholar]
- Peterson L. R., Hamernyik P., Bird T. D., Labbé R. F. Erythrocyte uroporphyrinogen I synthase activity in diagnosis of acute intermittent porphyria. Clin Chem. 1976 Nov;22(11):1835–1840. [PubMed] [Google Scholar]
- Pimstone N. R., Blekkenhorst G., Eales L. Enzymatic defects in hepatic porphyria. Preliminary observations in patients with porphyria cutanea tarda and variegate porphyria. Enzyme. 1973;16(1):354–366. [PubMed] [Google Scholar]
- Romeo G. Enzymatic defects of hereditary porphyrias: an explanation of dominance at the molecular level. Hum Genet. 1977 Dec 23;39(3):261–276. doi: 10.1007/BF00295419. [DOI] [PubMed] [Google Scholar]
- Sassa S., Bernstein S. E. Levels of delta-aminolevulinate dehydratase, uroporphyrinogen-I synthase, and protoporphyrin IX in erythrocytes from anemic mutant mice. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1181–1184. doi: 10.1073/pnas.74.3.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Levere R. D., Kappas A. Studies on the inheritance of human erythrocyte delta-aminolevulinate dehydratase and uroporphyrinogen synthetase. Enzyme. 1973;16(1):326–333. doi: 10.1159/000459397. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Kappas A. Effect of lead and genetic factors on heme biosynthesis in the human red cell. Ann N Y Acad Sci. 1975 Apr 15;244:419–440. doi: 10.1111/j.1749-6632.1975.tb41546.x. [DOI] [PubMed] [Google Scholar]
- Strand L. J., Meyer U. A., Felsher B. F., Redeker A. G., Marver H. S. Decreased red cell uroporphyrinogen I synthetase activity in intermittent acute porphyria. J Clin Invest. 1972 Oct;51(10):2530–2536. doi: 10.1172/JCI107068. [DOI] [PMC free article] [PubMed] [Google Scholar]


