Abstract
Catabolism of amino acids via the Ehrlich pathway involves transamination to the corresponding α-keto acids, followed by decarboxylation to an aldehyde and then reduction to an alcohol. Alternatively, the aldehyde may be oxidized to an acid. This pathway is functional in Saccharomyces cerevisiae, since during growth in glucose-limited chemostat cultures with phenylalanine as the sole nitrogen source, phenylethanol and phenylacetate were produced in quantities that accounted for all of the phenylalanine consumed. Our objective was to identify the structural gene(s) required for the decarboxylation of phenylpyruvate to phenylacetaldehyde, the first specific step in the Ehrlich pathway. S. cerevisiae possesses five candidate genes with sequence similarity to genes encoding thiamine diphosphate-dependent decarboxylases that could encode this activity: YDR380w/ARO10, YDL080C/THI3, PDC1, PDC5, and PDC6. Phenylpyruvate decarboxylase activity was present in cultures grown with phenylalanine as the sole nitrogen source but was absent from ammonia-grown cultures. Furthermore, the transcript level of one candidate gene (ARO10) increased 30-fold when phenylalanine replaced ammonia as the sole nitrogen source. Analyses of phenylalanine catabolite production and phenylpyruvate decarboxylase enzyme assays indicated that ARO10 was sufficient to encode phenylpyruvate decarboxylase activity in the absence of the four other candidate genes. There was also an alternative activity with a higher capacity but lower affinity for phenylpyruvate. The candidate gene THI3 did not itself encode an active phenylpyruvate decarboxylase but was required along with one or more pyruvate decarboxylase genes (PDC1, PDC5, and PDC6) for the alternative activity. The Km and Vmax values of the two activities differed, showing that Aro10p is the physiologically relevant phenylpyruvate decarboxylase in wild-type cells. Modifications to this gene could therefore be important for metabolic engineering of the Ehrlich pathway.
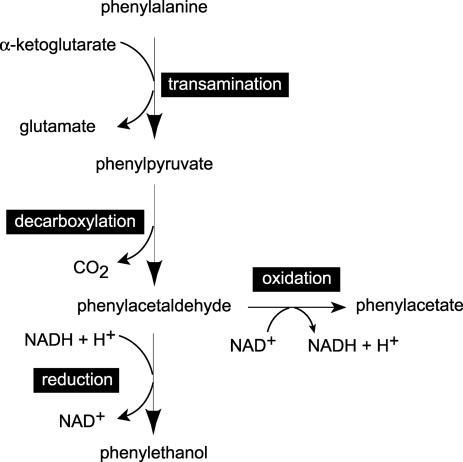
The yeast Saccharomyces cerevisiae can use a variety of amino acids as sole nitrogen sources, including three aromatic amino acids, l-tryptophan, l-phenylalanine, and l-tyrosine (10). The primary catabolic products are tryptophol, phenylethanol, and tyrosol, respectively, which are collectively known as fusel oils (32, 34, 40). Fusel oil formation from amino acids is assumed to proceed via the Ehrlich pathway by means of three enzyme-catalyzed reactions. In the case of phenylalanine, the amino acid is deaminated to phenylpyruvic acid and then decarboxylated to phenylacetaldehyde and reduced to phenylethanol (Fig. 1) (16).
FIG. 1.
Catabolism of phenylalanine via the Ehrlich pathway.
Phenylethanol, which has a rose-like aroma, is an important fragrance in the cosmetic industry (9, 19) and possesses organoleptic characteristics that contribute to the quality of beverages and foods (19, 22, 52). While chemically synthesized phenylethanol is a valuable compound, phenylethanol that is synthesized biologically is 250- to 300-fold more expensive (17). Various organisms, including S. cerevisiae, can produce phenylethanol (2, 18, 50), and optimization of production in S. cerevisiae has been the subject of recent research (41). Despite this interest, the production of phenylethanol by S. cerevisiae is poorly characterized both genetically and biochemically.
A critical step in phenylethanol production is the decarboxylase reaction, which is the first specific step in phenylalanine catabolism (Fig. 1). The S. cerevisiae genome contains five candidate genes that could encode phenylpyruvate decarboxylase activity. These are PDC1, PDC5, and PDC6, as well as two open reading frames, YDR380w and YDL080c, which are also thought to encode thiamine diphosphate-dependent decarboxylases (28). PDC1, PDC5, and PDC6 encode the major activity for pyruvate decarboxylation (27). Both the activity and nature of this enzyme activity in yeast have been extensively studied (for reviews see references 21 and 39). In the catabolism of branched-chain amino acids, the PDC genes contribute to fusel alcohol production, but a PDC-independent activity also exists (42). The PDC homologs Ydl080cp and Ydr380wp contribute to the catabolism of isoleucine (12), and the protein encoded by YDL080c is important for leucine catabolism, while valine catabolism involves several pyruvate decarboxylase isozymes (13, 14). For the aromatic amino acids, decarboxylases for the derived α-keto acids have not been described. However, Iraqui et al. (30) found that the YDR380w/ARO10 open reading frame was transcriptionally induced when cells were grown in the presence of tryptophan with urea as a nitrogen source.
In this study, our objective was to identify the gene(s) that encodes phenylpyruvate decarboxylase(s) in S. cerevisiae. We hypothesized that one or more of the five S. cerevisiae genes for thiamine diphosphate-dependent decarboxylases encode phenylpyruvate decarboxylation activity. By using a combination of genetic, genomic, physiological, and biochemical approaches, we found that YDR380w/ARO10 encodes the main physiologically relevant phenylpyruvate decarboxylase activity in wild-type S. cerevisiae. Additionally, we partially characterized an alternative activity that requires the presence of both YDL080c and one of the pyruvate decarboxylase genes.
MATERIALS AND METHODS
Strains.
The S. cerevisiae strains used in this study are listed in Table 1. Strains were constructed by using standard yeast media and genetic techniques (3, 51). The kanamycin resistance cassette was amplified by using the pUG vector as the template (24).
TABLE 1.
Strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| CEN.PK113-7D | MATaMAL2-8c SUC2 | P. Köttera |
| CEN.PK555-4A | MATaMAL2-8c SUC2 ydr380w::loxP-Kan-loxP | This study |
| CEN.PK632-3B | MATα MAL2-8c SUC2 ydl080c::loxP-Kan-loxP ydr380w::loxP-Kan-loxP | This study |
| CEN.PK 608-4B | MATaMAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::loxP ydl080c::loxP-Kan-loxP | This study |
| CEN.PK609-11A | MATaMAL2-8c SUC2 pdc1::loxP pdc5::loxP pdc6::loxP ydr380w::loxP-Kan-loxP | This study |
| CEN.PK 689-6C | MATaMAL2-8c SUC2 pdc5::loxP-Kan-loxP ydr380w::loxP-Kan-loxP | This study |
Institut für Mikrobiologie der J. W. Goethe Universität, Frankfurt, Germany.
Chemostat cultivation.
Aerobic chemostat cultivation was performed at 30°C in 1-liter (working volume) laboratory fermentors (Applikon, Schiedam, The Netherlands) at a stirrer speed of 800 rpm and pH 5.0 with a dilution rate of 0.10 h−1, as described by Van den Berg et al. (44). The pH was kept constant by using an ADI 1030 biocontroller (Applikon) and automatic addition of 2 M KOH. The fermentor was flushed with air at a flow rate of 0.5 liter min−1 by using a Brooks 5876 mass flow controller (Brooks Instruments, Veenendaal, The Netherlands). The dissolved oxygen concentration was continuously monitored with an Ingold model 34 100 3002 probe (Mettler-Toledo, Greifensee, Switzerland) and was more than 50% of air saturation.
Carbon-limited steady-state chemostat cultures of both wild-type and mutant strains were grown on the mineral medium described by Verduyn et al. (47) containing 7.5 g of glucose liter−1 as carbon source and either 5.0 g of (NH4)2SO4 liter−1 or 5.0 g of phenylalanine liter−1 as the sole nitrogen source. When phenylalanine was the sole nitrogen source, the amino acid solution was sterilized separately by autoclaving it before addition to the medium, and the absence of (NH4)2SO4 was compensated for by addition of equimolar amounts of K2SO4. For chemostat cultivation of pyruvate decarboxylase-negative strains, 7.1 g of glucose liter−1 and 0.38 g of acetate liter−1 (5% acetate on a carbon basis) were used as carbon sources to overcome the C2 requirement of PDC-negative strains (20).
For anaerobic cultivation, media were supplemented with the anaerobic growth factors ergosterol and Tween 80 (10 and 420 mg liter−1, respectively), and the glucose concentration was increased to 25 g liter−1 (49). To maintain anaerobic conditions, both the culture vessel and inflowing media were sparged with nitrogen gas at a flow rate of 0.5 liter min−1, and the fermentors were equipped with Norprene tubing and butyl rubber septa to prevent O2 diffusion into the cultures.
Shake flask cultivation.
Growth rate experiments were performed in 500-ml flasks containing 100 ml of medium, which were incubated at 30°C on an orbital shaker set at 200 rpm. When growth rates on phenylalanine were determined, mineral medium (47) with 5.0 g of phenylalanine liter−1 as the sole nitrogen source was used. The pH was adjusted to 6.0 with 2 M KOH, and then the medium was filter sterilized with a MediaKap-5 filter (Spectrum Europe, Breda, The Netherlands) with a pore size of 0.2 μm. Sterile glucose was added to a final concentration of 2% as the carbon source.
Preparation of cell extracts.
For preparation of cell extracts, culture samples were harvested by centrifugation, washed twice with 10 mM potassium phosphate buffer (pH 7.5) containing 2 mM EDTA, concentrated fourfold, and stored at −20°C. Before cell breakage, the samples were thawed at room temperature, washed, and resuspended in 100 mM potassium phosphate buffer (pH 7.5) containing 2 mM MgCl2 and 2 mM dithiothreitol. Extracts were prepared by sonicating preparations with 0.7-mm-diameter glass beads at 0°C for 2 min at 0.5-min intervals with an MSE sonicator (150-W output, 7-μm peak-to-peak amplitude). Unbroken cells and debris were removed by centrifugation at 4°C for 20 min at 36,000 × g. The purified cell extracts were used for enzyme assays.
Enzyme assays.
Pyruvate decarboxylase activity was measured as described by Flikweert et al. (20). Phenylpyruvate decarboxylase activity was measured at 30°C immediately after preparation of cell extracts by using a coupled reaction. Activity was measured by monitoring the reduction of NAD+ at 340 nm in the presence of excess aldehyde dehydrogenase from yeast. The reaction mixtures (total volume, 1 ml) contained 70 mM KH2PO4/K2HPO4 buffer (pH 7.0), 2 mM NAD+, 0.2 mM thiamine diphosphate, 0.35 U of yeast aldehyde dehydrogenase (Sigma-Aldrich, Zwijndrecht, The Netherlands) (dissolved in 1 mM dithiothreitol), and 2 mM phenylpyruvic acid to initiate the reaction. The reaction rates were linearly proportional to the amount of cell extract added. For determination of Km and Vmax, the reaction mixture remained the same while the substrate concentration was adjusted from 0.125 to 5 mM.
Analytical procedures.
Measurements of biomass, metabolites from culture supernatants, and gasses were obtained as previously described (5). The metabolites of phenylalanine catabolism were analyzed with a high-performance liquid chromatograph fitted with an Alltech Platinum EPS C18 column (pore size, 0.01 μm; particle size, 5 μm; Alltech Nederland, Breda, The Netherlands). The mobile phase was phosphate buffer (pH 2.7) with a 5 to 40% acetonitrile gradient at a flow rate of 1 ml min−1 at room temperature. The error introduced by the measurement technique was less than 5%.
Microarrays.
DNA microarray analyses were performed with S98 Yeast GeneChip arrays from Affymetrix (Santa Clara, Calif.) as previously described (37). Cells were transferred directly from chemostats into liquid nitrogen and processed according to the manufacturer's instructions (Affymetrix technical manual). Data analyses were performed with the following Affymetrix software packages: Microarray Suite v5.0, MicroDB v3.0, and Data Mining Tool v3.0. Microsoft Excel with the Significance Analysis of Microarrays (SAM v1.12) (43) plug-in was used for further statistical analyses.
RESULTS
Physiology of S. cerevisiae grown with phenylalanine as the sole nitrogen source.
Phenylpyruvate, phenylacetate, and phenylethanol were all detected in the supernatants of aerobic, glucose-limited chemostat cultures grown with phenylalanine as the sole nitrogen source but not in cultures grown with ammonia as the sole nitrogen source (Table 2). These three catabolites and the residual phenylalanine in the culture medium could account for all the phenylalanine supplied in the feed, indicating that no other metabolites were formed from phenylalanine (Table 2). During anaerobic growth the metabolite profile of the supernatant was different from that during aerobic growth. No phenylacetate was detected, and the phenylalanine supplied could be accounted for by the amount of residual phenylalanine, phenylpyruvate, and phenylethanol (Table 2).
TABLE 2.
Characteristics of aerobic and anaerobic glucose-limited chemostat cultures (dilution rate, 0.1 h−1) of wild-type S. cerevisiae CEN.PK 113-7D with phenylalanine or ammonia as the sole nitrogen sourcea
| Culture | Physiological characteristics
|
Metabolite profiles of culture supernatants
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N source | Aeration | Biomass yield (g g of glucose−1) | qglucoseb | qO2c | qethanold | qCO2e | Residual phenylalanine concn (mM) | Phenylpyruvate concn (mM) | Phenylacetate concn (mM) | Phenylethanol concn (mM) | Recovery of phenyl C skeleton (%)f |
| (NH4)2SO4 (38 mM) | Aerobicg | 0.49 ± 0.01 | 1.1 ± 0.0 | 2.8 ± 0.3 | NAh | 2.8 ± 0.3 | NA | <0.1 | <0.1 | <0.1 | NA |
| Phenylalanine (30 mM) | Aerobic | 0.3 ± 0.01 | 1.7 ± 0.2 | 7.1 ± 0.3 | NA | 7 ± 0.4 | 19.2 ± 4.6 | <0.1 | 11.0 ± 3.1 | 1.7 ± 0.2 | 106 ± 7 |
| (NH4)2SO4 (38 mM) | Anaerobici | 0.1 ± 0.0 | 5.6 ± 0.2 | NA | 8.5 ± 0.0 | 8.7 ± 0.6 | NA | <0.1 | <0.1 | <0.1 | NA |
| Phenylalanine (30 mM) | Anaerobic | 0.07 ± 0.0 | 8.1 ± 0.2 | NA | 12.4 ± 0.3 | 15.2 ± 0.4 | 21.7 ± 1.5 | 0.12 ± 0.0 | <0.1 | 8.3 ± 0.2 | 100 ± 5 |
All values are means ± maximal deviations derived from two independent experiments.
Rate of glucose consumption, expressed in millimoles of glucose consumed per gram of biomass per hour.
Rate of O2 consumption, expressed in millimoles of O2 consumed per gram of biomass per hour.
Rate of ethanol production, expressed in millimoles of ethanol produced per gram of biomass per hour.
Rate of CO2 production, expressed in millimoles of CO2 produced per gram of biomass per hour.
Amount of phenylalanine consumed (sum of phenylalanine catabolites in spent medium plus 0.17 mM per biomass for protein synthesis [33]) expressed as a percentage of the phenylalanine in the feed.
Data from reference 5.
NA, not applicable.
Data are from reference 46.
Phenylpyruvate decarboxylase activity (53 ± 4 nmol mg of protein−1 min−1) was detected in cell extracts of aerobic, glucose-limited chemostat cultures grown with phenylalanine as the sole nitrogen source but not in extracts of cultures grown with ammonia as the sole nitrogen source. Thus, we concluded that phenylpyruvate decarboxylase was regulated and was induced in cultures with phenylalanine as the sole nitrogen source.
Identification of a putative phenylpyruvate decarboxylase gene.
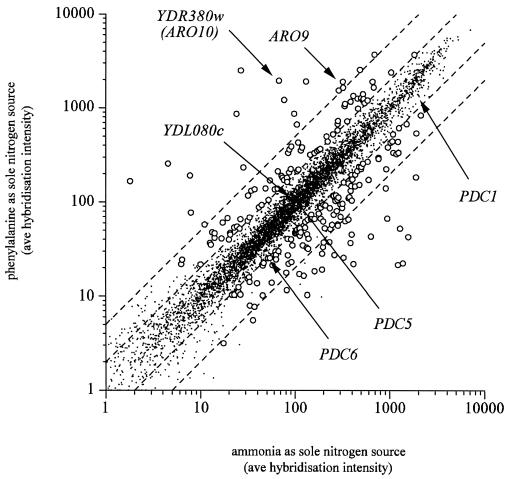
We used DNA microarrays to compare the transcriptomes of wild-type cells grown in glucose-limited chemostats with phenylalanine as the sole nitrogen source and the transcriptomes of cells grown with ammonia as the sole nitrogen source. (The entire data set is available at http://www.phepdc.bt.tudelft.nl.) We identified 89 transcripts that were expressed at a significantly higher level when phenylalanine was the sole nitrogen source and 146 transcripts that were expressed at a significantly higher level when ammonia was the sole nitrogen source. Of the five thiamine diphosphate-dependent decarboxylases, YDR380w/ARO10 was the only transcript whose level increased (it increased 30-fold) when cells were grown on phenylalanine (Fig. 2). YDR380w/ARO10 was therefore considered a strong candidate to encode phenylpyruvate decarboxylase activity.
FIG. 2.
Genome-wide transcription levels for wild-type S. cerevisiae grown on ammonia as the sole nitrogen source plotted versus genome-wide transcription levels for wild-type S. cerevisiae grown on phenylalanine as the sole nitrogen source: average signals in microarrays from glucose-limited chemostat cultures grown with ammonia (n = 4) and phenylalanine (n = 3) as the sole nitrogen sources for all 6,383 yeast open reading frames. The open circles represent genes whose expression was significantly different for the two conditions, while the solid dots represent transcripts whose expression was not significantly altered. The diagonal lines are the boundaries for two- and fivefold differences in signals between conditions. The full data set is available at http://www.phepdc.bt.tudelft.nl.
To test this hypothesis, we grew an aro10 deletion strain (CEN.PK 555-4A) in shake flasks with phenylalanine as the sole nitrogen source. The maximum specific growth rate of this strain was three- to fourfold lower than that of the wild-type strain in the same medium (data not shown). In contrast, the growth rates of the two strains were similar when ammonia was the sole nitrogen source, indicating that the reduced growth rate of the mutant was related to phenylalanine catabolism. In cell extracts of the aro10 knockout strain grown with phenylalanine as the sole nitrogen source, there was no detectable phenylpyruvate decarboxylase activity. In contrast, there was measurable activity (22 ± 1 nmol mg of protein−1 min−1) in wild-type extracts grown under the same conditions. These data suggest that ARO10 is both necessary and sufficient for phenylpyruvate decarboxylase activity in shake flask cultures of S. cerevisiae.
Identification of an alternative phenylpyruvate decarboxylase activity.
The supernatant profile and enzyme activities of the aro10 mutant strain were determined when cells were grown in aerobic, glucose-limited chemostat cultures at a dilution rate of 0.10 h−1 with phenylalanine as the sole nitrogen source. The profiles of phenylalanine catabolites in the culture supernatants were similar for wild-type strain CEN.PK113-7D and mutant strain CEN.PK555-4A (Table 3). However, in contrast to the situation in shake flask cultures, the measured phenylpyruvate decarboxylase activity of the aro10 mutant grown with phenylalanine as the sole nitrogen source was fourfold higher than that of the wild-type strain. Thus, there was an alternative phenylpyruvate decarboxylase activity in chemostat-grown cells that was not expressed during growth in shake flasks. Indeed, a strain in which all thiamine diphosphate-dependent decarboxylase genes except ARO10 were deleted (CEN.PK608-4B) still exhibited phenylpyruvate decarboxylase activity and had phenylpyruvate catabolites in the culture supernatants when it was grown in chemostat cultures with phenylalanine as the sole nitrogen source (Table 3).
TABLE 3.
Enzyme activities and metabolite profiles of aerobic glucose-limited chemostat cultures of S. cerevisiae strains grown with phenylalanine as the sole nitrogen source (dilution rate, 0.1 h−1)a
| Strain | Relevant genotype | Enzyme activities (nmol mg of protein−1 min−1)
|
Conc of phenylalanine catabolites in culture supernatants (mM)
|
|||
|---|---|---|---|---|---|---|
| Pyruvate decarboxylase | Phenylpyruvate decarboxylase | Phenylpyruvate | Phenylacetate | Phenylethanol | ||
| CEN.PK 113-7D | PDC1 PDC5 PDC6 YDR380w YDL080cb | 140 ± 2.0 | 53 ± 3.5 | <0.1 | 11 ± 3.1 | 1.7 ± 0.2 |
| CEN.PK 555-4A | PDC1 PDC5 PDC6 ydr380w YDL080c | 180 ± 2.0 | 220 ± 17 | 0.75 ± 0.40 | 8.9 ± 0.18 | 2.5 ± 0.0 |
| CEN.PK 632-3B | PDC1 PDC5 PDC6 ydr380w ydl080c | 540 ± 55 | <2.0 | 7.4 ± 0.12 | <0.1 | 0.81 ± 0.0 |
| CEN.PK 609-11A | pdc1 pdc5 pdc6 ydr380w YDL080c | <10 | <2.0 | 6.5 ± 1.0 | <0.1 | <0.1 |
| CEN.PK 608-4B | pdc1 pdc5 pdc6 YDR380w ydl080c | <10 | 40 ± 1.5 | 0.41 ± 0.1 | 7.4 ± 2 | 3.5 ± 0.3 |
All values are means ± maximal deviations derived from two independent experiments.
YDR380w is ARO10, and YDL080c is THI3.
The S. cerevisiae genome contains four PDC-like open reading frames (PDC1, PDC5, PDC6, and YDL080c). Potentially, each of these could encode an enzyme that could decarboxylate phenylpyruvate in the absence of ARO10. The PDC1, PDC5, and PDC6 gene products are extremely similar (79 to 86% sequence identity for all pairwise comparisons), and each product has pyruvate decarboxylase activity (25, 26). The fourth homologous open reading frame, YDL080c, is less well characterized but has been implicated in the decarboxylation of the branched-chain 2-oxo acids and the regulation of genes involved in thiamine metabolism (12-14, 36).
Both a double-deletion strain (aro10 ydl080c; CEN.PK 632-3B) and a quadruple-deletion strain (pdc1 pdc5 pdc6 aro10; CEN.PK 609-11A) were grown in aerobic, glucose-limited chemostat cultures with phenylalanine as the sole nitrogen source. Cell extracts from these strains contained no measurable phenylpyruvate decarboxylase activity (Table 3). These assay data were supported by metabolite profile analyses of the culture supernatants, since phenylacetate was undetectable in both cultures and only small amounts of phenylethanol were found in the culture of the double-deletion strain (aro10 ydl080c) (Table 3). The quadruple-deletion strain containing only YDL080c (CEN.PK 609-11A) showed no evidence of the decarboxylase activities examined (Table 3), demonstrating that Ydl080cp alone could not decarboxylate phenylpyruvate or pyruvate. This result also indicated that it is unlikely that there are any other genes that encode the alternative decarboxylase activity. Instead, the phenotypes of the multiple-deletion strains indicate that the ARO10-independent phenylpyruvate decarboxylase activity requires the presence of both YDL080c and at least one of the PDC genes (Table 3).
Physiological relevance of S. cerevisiae phenylpyruvate decarboxylase activities.
We determined the Km and Vmax values for Aro10p and the alternative phenylpyruvate decarboxylase activity. Cell extracts from a strain containing only the ARO10-encoded activity (CEN.PK 608-4B) were compared with extracts from a strain containing only the alternative activity involving Ydl080cp and one or more of the pyruvate decarboxylases (CEN.PK 555-4A). The phenylpyruvate decarboxylase activities of extracts of the wild-type strain and the quadruple-deletion strain (containing only Aro10p) displayed Michaelis-Menten saturation kinetics. Reduction of the substrate concentration in the assay mixture to less than 0.5 mM resulted in altered enzyme activity, from which the Km and Vmax values were estimated (Table 4). The Vmax values for the activities of these two extracts were similar (Table 3). However, the extract from the quadruple mutant (CEN.PK 608-4B) had a slightly higher affinity (Km, 0.062 ± 0.005 mM) for the substrate phenylpyruvate than the wild type had (Km, 0.10 ± 0.001 mM). Extracts from the strain lacking ARO10 alone (CEN.PK555-4A) resulted in a sigmoidal curve in a plot of substrate concentration versus velocity. This result is consistent with previous observations of cooperativity of pyruvate decarboxylase in the presence of phosphate (6). This PDC-like behavior is consistent with the genetic data, according to which at least one PDC gene is required for the Aro10p-independent phenylpyruvate decarboxylase activity.
TABLE 4.
Km and Vmax valuesa determined for phenylpyruvate decarboxylase activity in cell extracts from three S. cerevisiae strains grown in aerobic glucose-limited chemostats with phenylalanine as the sole nitrogen source (dilution rate, 0.1 h−1)
| Strain | Relevant genotype | Km (mM) | Vmax (nmol mg of protein−1 min−1) |
|---|---|---|---|
| CEN.PK 113-7D | PDC1 PDC5 PDC6 YDR380w YDL080c | 0.10 ± 0.001 | 54 ± 6 |
| CEN.PK 608-4B | pdc1 pdc5 pdc6 YDR380w ydl080c | 0.062 ± 0.005 | 38 ± 3 |
| CEN.PK 555-4A | PDC1 PDC5 PDC6 ydr380w YDL080c | 0.48 ± 0.08 | 240 ± 28 |
The values are means ± maximal deviations derived from two independent experiments for CEN.PK 113-7D and CEN.PK 608-4B and from three independent experiments for CEN.PK 555-4A.
In supernatants from wild-type cultures grown with phenylalanine as the sole nitrogen source, all of the phenylalanine consumed was recovered as either phenylacetate or phenylethanol (Table 2). By using the sum of the concentrations of phenylalanine catabolites in the supernatant and the biomass concentration in the culture vessel (2.23 g liter−1), the specific rate of catabolite production was calculated to be 0.57 mmol g of biomass−1 h−1. Since the catabolites were derived from phenylpyruvate decarboxylation, the steady-state flux through the decarboxylase enzyme must also have been 0.57 mmol g of biomass−1 h−1 (or 9.5 nmol mg of biomass−1 min−1). Since the estimated soluble protein content is 0.33 g g of biomass−1 for yeast (38), this rate was converted to a specific activity of 29 nmol mg of protein−1 min−1. If values were substituted into the Michaelis-Menten equation and the Vmax and Km values obtained for wild-type cell extracts were used, the substrate concentration inside the cells was 0.11 mM. This value is the same as the Km determined for wild-type extracts and is slightly higher than the Km found for the mutant containing Aro10p only (CEN.PK 608-4B) (Table 4). The Km of the activity from cells lacking ARO10 was five- to eightfold higher than that from strains that contained a wild-type ARO10 allele (Table 4). Therefore, at deduced intracellular phenylpyruvate concentrations of ca. 0.1 mM, this compound is preferentially catabolized by the ARO10-encoded activity.
Involvement of PDC5 in ARO10-independent phenylpyruvate decarboxylation.
We used a recently compiled transcriptome database for cells grown under four different nutrient limitation regimens (5) to evaluate the correlation between the expression of thiamine diphosphate-dependent decarboxylase genes and phenylpyruvate decarboxylase activity in wild-type S. cerevisiae (Table 5) (for the complete data sets accompanying these arrays see reference 5). Phenylpyruvate decarboxylase was detected only during aerobic growth under nitrogen limitation conditions with ammonia as the nitrogen source and glucose as the carbon source and when there was phosphate-limited growth with ammonia as the nitrogen source and glucose as the carbon source (Table 5). Under these conditions, the levels of the ARO10 transcript were negligible, indicating that the observed phenylpyruvate decarboxylase activity was due to the ARO10-independent activity discussed above. In all four cultures, low but significant levels of the YDL080c transcript were detected. However, phenylpyruvate decarboxylase was detected in cell extracts when PDC5 was transcribed at high levels but not when PDC1 (glucose limitation) or PDC6 (sulfur limitation) was the predominantly transcribed PDC gene. Furthermore, the levels of the PDC5 transcript in glucose-limited chemostat cultures of the aro10 strain grown with phenylalanine as the sole nitrogen source were over eightfold higher than the levels in similar cultures of the wild type, while the levels of the PDC1, PDC6, and YDL080c transcripts differed by less than twofold (data not shown).
TABLE 5.
Enzyme activities and transcript levels of the thiamine diphosphate-dependent decarboxylases of S. cerevisiae under four nutrient limitation regimens in chemostat culturesa
| Growth limitation | Enzyme activities (nmol mg of protein−1 min−1)
|
Transcript levelb
|
|||||
|---|---|---|---|---|---|---|---|
| Pyruvate decarboxylase | Phenylpyruvate decarboxylase | PDC1 | PDC5 | PDC6 | YDR380w | YDL080c | |
| Carbon | 580 ± 0.0 | <2.0 | 1,900 ± 450 | 95 ± 5 | 66 ± 44 | 67 ± 4 | 92 ± 9 |
| Nitrogen | 2,300 ± 25 | 10 ± 0.05 | 2,500 ± 190 | 1,700 ± 87 | 160 ± 30 | <12 | 250 ± 34 |
| Phosphorus | 1,800 ± 50 | 4 ± 0.35 | 2,400 ± 350 | 840 ± 33 | 37 ± 7 | 14 ± 1 | 120 ± 27 |
| Sulfur | 520 ± 30 | <2.0 | 2,000 ± 240 | 170 ± 36 | 1,900 ± 240 | <12 | 110 ± 16 |
The cultivation conditions and transcript data are from reference 5.
The transcript levels are data for triplicate arrays sampled from three independent chemostats.
To test the hypothesis that PDC5 but not PDC6 or PDC1 contributes to the alternative phenylpyruvate decarboxylase activity, we grew a pdc5 aro10 double-deletion strain (CEN.PK 689-6C) in aerobic, glucose-limited chemostat cultures with phenylalanine as the sole nitrogen source. Cell extracts of steady-state chemostat cultures did not exhibit significant phenylpyruvate decarboxylase activity (<2.5 nmol mg of protein−1 min−1), and <10% of the phenylpyruvate formed was converted to phenylacetate. The pyruvate decarboxylase activity in cell extracts of these cultures was 565 ± 45 nmol mg of protein−1 min−1, and preliminary experiments indicated that PDC1 but not PDC6 was expressed transcriptionally (as determined with a single measurement) (data not shown). However, when the cultures were grown for more than eight generations, the phenylpyruvate decarboxylase activity gradually increased and, after approximately 15 generations, reached specific activities in vitro that exceeded those of wild-type cultures and were similar to that of the aro10 deletion strain (265 ± 10 nmol mg of protein−1 min−1). This increase was reflected in the metabolite profile of the growth medium, which was similar to that of the aro10 deletion strain growth medium (data not shown).
DISCUSSION
Products of phenylalanine catabolism in S. cerevisiae.
When wild-type S. cerevisiae was grown in glucose-limited chemostats with phenylalanine as the sole nitrogen source, phenylethanol and phenylacetate could account for all of the phenylalanine consumed from the feed (Table 2). This result is consistent with the involvement of the Ehrlich pathway in phenylalanine catabolism (Fig. 1). In anaerobic chemostat cultures there was an almost stoichiometric conversion of phenylalanine to phenylethanol (Table 2) that probably reflected the altered redox state of the cells to favor the reductive branch of the Ehrlich pathway over the oxidative, phenylacetate-yielding branch (Fig. 1). The presence of these catabolites in the medium was reflected in the physiological growth parameters measured.
When phenylalanine was used instead of ammonia as the sole nitrogen source, the biomass yield on glucose (expressed in grams [dry weight] of biomass per gram of glucose consumed) of wild-type S. cerevisiae was substantially lower (Table 2). In addition, higher rates of carbon dioxide production and, in the aerobic cultures, oxygen consumption accompanied the reduced biomass yield. It has been hypothesized that these changes are indicative of uncoupling caused by enhanced proton cycling via the weak acid phenylacetate (48) and by the stimulating effect of phenylethanol on membrane fluidity (29, 41).
Decarboxylation of phenylpyruvate is not a prerequisite for the transamination of phenylalanine and, hence, utilization of this compound as a sole nitrogen source (Fig. 1). Nevertheless, growth with phenylalanine as the sole nitrogen source in shake flask cultures was substantially slower for an aro10 mutant, which lacked detectable phenylpyruvate decarboxylase activity, than for the wild type. This difference suggests that the physiological role of phenylpyruvate decarboxylation may be to prevent the accumulation of growth-inhibiting concentrations of phenylpyruvate.
Transcriptional regulation of phenylalanine catabolism.
Transcriptional regulation of genes in response to phenylalanine occurs via at least two routes in S. cerevisiae. The first route involves an intracellular sensor of aromatic amino acids that regulates genes through the transcriptional activator Aro80p (30), and the second route involves a sensor of external amino acids (31). We identified the binding site for Stp1p (a transcriptional regulator downstream of extracellular amino acid sensing [35]), which was overrepresented in the promoters of genes whose transcription was greater when phenylalanine was the nitrogen source (45). An additional element known to bind the GATA family of transcriptional regulators (8) also was found, indicating that there was control via the general response to the use of a nonpreferred nitrogen source (nitrogen catabolite repression [NCR]). Only nine gene promoters in the genome contain an exact match with the proposed binding site for Aro80p (direct repeat of 5′-T[A/T][A/G]CCG-3′ separated by four nucleotides) (30). Among the nine genes, there are four pairs of divergently transcribed genes and one gene without a shared promoter. There were three genes with significantly higher transcript levels in chemostat cultures containing phenylalanine instead of ammonia as the sole nitrogen source (ARO9, ARO10, and ESPB6, exhibiting 6-, 30-, and 2.5-fold changes in transcript levels, respectively). The promoters of these three genes contained the binding site repeat in the forward direction. If this promoter element operates unidirectionally, it could explain why five of the remaining genes, which have the reverse complement sequence in their promoters, were not regulated in a similar manner. Based on phenotypic analysis of an aro80 deletion strain, it is not surprising that the domain of Aro80p's control is limited to the degradation of aromatic amino acids (1, 30). Similar regulatory events have also been reported in other microorganisms (4, 11, 23) and probably result from the need to separate phenylalanine biosynthesis from phenylalanine catabolism since the two pathways share phenylpyruvate as an intermediate and are thought to be colocalized in the cytosol.
The wider effects on the transcriptome could be caused by the activity of NCR due to the presence of an aromatic amino acid as the sole nitrogen source. Many genes (including a number of regulated genes according to our data that are required for nutrient transport) are under the control of this regulon. However, previous results have shown that NCR does not directly regulate expression of ARO9 or ARO10 (30). Rather, NCR modulates the expression of these genes indirectly by preventing Aro80p-dependent induction by inducer exclusion. Thus, cells cofed ammonia and an aromatic amino acid should preferentially catabolize ammonia by preventing uptake of the aromatic amino acid. Conversely, when ammonia is limiting for growth (or absent), this general repression is relieved, which allows uptake and assimilation of amino acids for use as nitrogen sources. (See supplementary material at http://www.phepdc.bt.tudelft.nl for all gene changes.)
Substrate specificity of thiamine diphosphate-dependent decarboxylases.
Of the five thiamine diphosphate-dependent decarboxylase genes in S. cerevisiae, the ARO10 transcript was the only transcript changed, and the level was 30-fold higher during growth on phenylalanine as the sole nitrogen source than during growth on ammonia as the sole nitrogen source. Two lines of evidence confirm that ARO10 encodes an active phenylpyruvate decarboxylase: (i) the clear phenotype of an aro10 null mutant in shake flask cultures grown with phenylalanine as the sole nitrogen source and (ii) the phenotype of a quadruple pdc1 pdc5 pdc6 ydl080c mutant in chemostat cultures grown with phenylalanine as the sole nitrogen source.
An alternative, ARO10-independent phenylpyruvate decarboxylase activity also was observed in chemostat cultures of the aro10 null mutant. This activity was not detectable in an aro10 ydl080c double mutant. The YDL080c product exhibits strong sequence similarity with known thiamine diphosphate-dependent decarboxylases and has a regulatory role in thiamine metabolism (7). Our data show that Ydl080cp cannot decarboxylate phenylpyruvate by itself. However, the combined presence of Ydl080cp and a pyruvate decarboxylase is required for the ARO10-independent phenylpyruvate decarboxylase activity. We have recently obtained evidence that a similar situation exists for the branched-chain 2-oxo acids that are formed during the catabolism of leucine, valine, and isoleucine (M. A. Morais and Z. Vuralhan, unpublished data). Analysis of transcript levels in wild-type cultures, as well as physiological analysis of a pdc5 aro10 strain, indicated that Pdc5p is primarily involved in the ARO10-independent, YDL080c-dependent phenylpyruvate decarboxylase activity. However the reappearance of phenylpyruvate decarboxylase activity of a pdc5 aro10 double mutant after prolonged chemostat cultivation suggests that only minor genetic changes allow another PDC gene to take over this role. We have not yet identified the nature of these mutations.
Part of the regulation of the different decarboxylases may occur at the level of transcription through a mechanism in which Thi3p acts as a sensor of intracellular thiamine diphosphate (7). In the presence of phenylalanine, the levels of the ARO10 transcript were among the highest 3% of the levels of transcripts of transcribed genes in wild-type cells. Deletion of this gene would probably alter the levels of intracellular thiamine diphosphate and trigger a signal via Thi3p to control transcription. However, the poor correlation between the transcript level and enzyme activity (Table 5) indicates that posttranscriptional regulation of the decarboxylase activities also occurs. An attractive model for this posttranscriptional regulation depends upon the in vivo tetrameric form of pyruvate decarboxylase (21). If the Pdc-like proteins can form heterotetramers, the resulting decarboxylase activities may have different substrate specificities. In this model, Aro10p and Thi3p could combine with one or more of the PDC-encoded proteins to produce enzymes that decarboxylate the α-keto acids produced during catabolism of the aromatic and branched-chain amino acids. This hypothesis can be tested with reconstitution experiments performed with different amounts and combinations of the purified proteins.
This study increased our understanding of phenylalanine catabolism in S. cerevisiae and illustrated the power of combining genome-wide transcript analyses with biochemical and genetic techniques to untangle functionally redundant enzyme activities. To date, analyses of single and multiple knockout mutants have proven to be insufficient to identify singular roles for the thiamine diphosphate-dependent decarboxylases in amino acid catabolism (12-15, 20). Our results provide new insight into the complexity of the regulation of substrate specificity of these decarboxylases, and they also provide a good basis for targeted metabolic engineering of phenylalanine catabolism.
ADDENDUM
While this manuscript was under review, Dickinson et al. (15) reported on the catabolism of phenylalanine to phenylethanol and the catabolism of tryptophan to tryptophol in S. cerevisiae. Using 13C nuclear magnetic resonance spectroscopy, gas chromatography-mass spectrometry, and a range of mutants, these authors showed that Aro10p can catalyze the decarboxylation of phenylpyruvate to phenylacetaldehyde and the decarboxylation of indolepyruvate to indolacetaldehyde and that, in the absence of an active aro10 gene, pyruvate decarboxylases are involved in phenylpyruvate decarboxylation.
Acknowledgments
This work was financially supported by the Board of the Delft University of Technology (BEO program), the Dutch Ministry of Economic Affairs, and the Kluyver Centre for Genomics of Industrial Fermentation. M.A.M. received support from Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq).
We thank Hans van Dijken for helpful comments during preparation of the manuscript and Peter Kötter for providing strains. The pdc1 pdc5 pdc6 triple-deletion strain was used with the permission of J. Lievense, Tate & Lyle/A.E. Staley Manufacturing Company.
REFERENCES
- 1.Akache, B., K. Wu, and B. Turcotte. 2001. Phenotypic analysis of genes encoding yeast zinc cluster proteins. Nucleic Acids Res. 29:2181-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albertazzi, E., R. Cardillo, S. Servi, and G. Zucchi. 1994. Biogeneration of 2-phenylethanol and 2-phenylethylacetate important aroma components. Biotechnol. Lett. 16:491-496. [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1989. Current protocols in molecular biology. John Wiley and Sons, New York, N.Y.
- 4.Barrowman, M. M., and C. A. Fewson. 1985. Phenylglyoxylate decarboxylase and phenylpyruvate decarboxylase from Acinetobacter calcoaceticus. Curr. Microbiol. 12:235-240. [Google Scholar]
- 5.Boer, V. M., J. H. de Winde, J. T. Pronk, and M. D. W. Piper. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus or sulfur. J. Biol. Chem. 278:3265-3274. [DOI] [PubMed] [Google Scholar]
- 6.Boiteux, A., and B. Hess. 1970. Allosteric properties of yeast pyruvate decarboxylase. FEBS Lett. 9:293-296. [DOI] [PubMed] [Google Scholar]
- 7.Burrows, R. J., K. L. Byrne, and P. A. Meacock. 2000. Isolation and characterization of Saccharomyces cerevisiae mutants with derepressed thiamine gene expression. Yeast 16:1497-1508. [DOI] [PubMed] [Google Scholar]
- 8.Bysani, N., J. R. Daugherty, and T. G. Cooper. 1991. Saturation mutagenesis of the UASNTR (GATAA) responsible for nitrogen catabolite repression-sensitive transcriptional activation of the allantoin pathway genes in Saccharomyces cerevisiae. J. Bacteriol. 173:4977-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark, G. S. 1990. Phenylethyl alcohol. Perfumer Flavorist 15:37-44. [Google Scholar]
- 10.Cooper, T. G. 1982. Nitrogen metabolism in Saccharomyces cerevisiae, p. 39-99. In J. N. Strathern, E. W. Jones, and J. R. Broach (ed.), The molecular biology of the yeast Saccharomyces: metabolism and gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.de Boer, L., W. Harder, and L. Dijkhuizen. 1988. Phenylalanine and tyrosine metabolism in the facultative methylotroph Nocardia sp. 239. Arch. Microbiol. 149:459-465. [Google Scholar]
- 12.Dickinson, J. R., S. J. Harrison, J. A. Dickinson, and M. J. Hewlins. 2000. An investigation of the metabolism of isoleucine to active amyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 275:10937-10942. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson, J. R., S. J. Harrison, and M. J. Hewlins. 1998. An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 273:25751-25756. [DOI] [PubMed] [Google Scholar]
- 14.Dickinson, J. R., M. M. Lanterman, D. J. Danner, B. M. Pearson, P. Sanz, S. J. Harrison, and M. J. Hewlins. 1997. A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J. Biol. Chem. 272:26871-26878. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson, J. R., L. E. J. Salgado, and M. J. E. Hewlins. 2003. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J. Biol. Chem. 278:8028-8034. [DOI] [PubMed] [Google Scholar]
- 16.Ehrlich, F. 1907. Über die Bedingungen der Fuselolbildung and über ihren Zusammenhang mit dem Eiweissaufbau der Hefe. Ber. Dtsch. Chem. Ges. 40:1027-1047. [Google Scholar]
- 17.Etschmann, M. M. W., W. Bluemke, D. Sell, and J. Schrader. 2002. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 59:1-8. [DOI] [PubMed] [Google Scholar]
- 18.Fabre, C. E., P. J. Blanc, and G. Goma. 1997. Screening of yeasts producing 2-phenylethylalcohol. Biotechnol. Tech. 11:523-525. [Google Scholar]
- 19.Fabre, C. E., P. J. Blanc, and G. Goma. 1998. Production of 2-phenylethyl alcohol by Kluyveromyces marxianus. Biotechnol. Prog. 14:270-274. [DOI] [PubMed] [Google Scholar]
- 20.Flikweert, M. T., L. Van Der Zanden, W. M. Janssen, H. Y. Steensma, J. P. van Dijken, and J. T. Pronk. 1996. Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12:247-257. [DOI] [PubMed] [Google Scholar]
- 21.Furey, W., P. Arjunan, L. Chen, M. Sax, F. Guo, and F. Jordan. 1998. Structure-function relationships and flexible tetramer assembly in pyruvate decarboxylase revealed by analysis of crystal structures. Biochim. Biophys. Acta 1385:253-270. [DOI] [PubMed] [Google Scholar]
- 22.Furia, T. E., and N. Bellanca. 1971. Fenaroli's handbook of flavor ingredients. CRC Press, Cleveland, Ohio.
- 23.Gopalakrishna, Y., T. K. Narayanan, and G. R. Rao. 1976. Biosynthesis of beta-phenethyl alcohol in Candida guilliermondii. Biochem. Biophys. Res. Commun. 69:417-422. [DOI] [PubMed] [Google Scholar]
- 24.Güldener, U., S. Heck, T. Fielder, J. Beinhauer, and J. H. Hegemann. 1996. A new efficient gene disruption cassette for repeated use in budding yeast. Nucleic Acids Res. 24:2519-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hohmann, S. 1991. Characterization of PDC6, a third structural gene for pyruvate decarboxylase in Saccharomyces cerevisiae. J. Bacteriol. 173:7963-7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hohmann, S. 1991. PDC6, a weakly expressed pyruvate decarboxylase gene from yeast, is activated when fused spontaneously under the control of the PDC1 promoter. Curr. Genet. 20:373-378. [DOI] [PubMed] [Google Scholar]
- 27.Hohmann, S., and H. Cederberg. 1990. Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. Eur. J. Biochem. 188:615-621. [DOI] [PubMed] [Google Scholar]
- 28.Hohmann, S., and P. A. Meacock. 1998. Thiamine metabolism and thiamine diphosphate-dependent enzymes in the yeast Saccharomyces cerevisiae: genetic regulation. Biochem. Biophys. Acta 1385:201-219. [DOI] [PubMed] [Google Scholar]
- 29.Ingram, L. O., and T. M. Buttke. 1984. Effects of alcohols on micro-organisms. Adv. Microb. Physiol. 25:253-300. [DOI] [PubMed] [Google Scholar]
- 30.Iraqui, I., S. Vissers, B. André, and A. Urrestarazu. 1999. Transcriptional induction by aromatic amino acids in Saccharomyces cerevisiae. Mol. Cell. Biol. 19:3360-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iraqui, I., S. Vissers, F. Bernard, J. O. de Craene, E. Boles, A. Urrestarazu, and B. André. 1999. Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol. Cell. Biol. 19:989-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kradolfer, P., P. Niederberger, and R. Hutter. 1982. Tryptophan degradation in Saccharomyces cerevisiae: characterization of two aromatic aminotransferases. Arch. Microbiol. 133:242-248. [DOI] [PubMed] [Google Scholar]
- 33.Lange, H. C., and J. J. Heijnen. 2001. Statistical reconciliation of the elemental and molecular biomass composition of Saccharomyces cerevisiae. Biotechnol. Bioeng. 75:334-344. [DOI] [PubMed] [Google Scholar]
- 34.Lingens, F. 1967. The biosynthesis of aromatic amino acids and its controls. Angew. Chem. Int. Ed. Engl. 6:811. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen, P. S., B. van den Hazel, T. Didion, M. de Boer, M. Jorgensen, R. J. Planta, M. C. Kielland-Brandt, and H. A. Andersen. 2001. Transcriptional regulation of the Saccharomyces cerevisiae amino acid permease gene BAP2. Mol. Gen. Genet. 264:613-622. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura, H., Y. Kawasaki, Y. Kaneko, K. Nosaka, and A. Iwashima. 1992. A positive regulatory gene, THI3, is required for thiamine metabolism in Saccharomyces cerevisiae. J. Bacteriol. 174:4701-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piper, M. D. W., P. Daran-Lapujade, C. Bro, B. Regenberg, S. Knudsen, J. Nielsen, and J. T. Pronk. 2002. Reproducibility of oligonucleotide microarray transcriptome analyses: an interlaboratory comparison using chemostat cultures of Saccharomyces cerevisiae. J. Biol. Chem. 277:37001-37008. [DOI] [PubMed] [Google Scholar]
- 38.Postma, E., C. Verduyn, W. A. Scheffers, and J. P. van Dijken. 1989. Enzymic analysis of the Crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 55:468-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pronk, J. T., H. Y. Steensma, and J. P. van Dijken. 1996. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 12:1607-1633. [DOI] [PubMed] [Google Scholar]
- 40.Sentheshanmuganathan, S. 1960. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 74:568-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stark, D., T. Munch, B. Sonnleitner, I. W. Marison, and U. von Stockar. 2002. Extractive bioconversion of 2-phenylethanol from l-phenylalanine by Saccharomyces cerevisiae. Biotechnol. Prog. 18:514-523. [DOI] [PubMed] [Google Scholar]
- 42.ter Schure, E. G., M. T. Flikweert, J. P. van Dijken, J. T. Pronk, and C. T. Verrips. 1998. Pyruvate decarboxylase catalyzes decarboxylation of branched-chain 2-oxo acids but is not essential for fusel alcohol production by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 64:1303-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van den Berg, M. A., P. de Jong-Gubbels, C. J. Kortland, J. P. van Dijken, J. T. Pronk, and H. Y. Steensma. 1996. The two acetyl-coenzyme A synthetases of Saccharomyces cerevisiae differ with respect to kinetic properties and transcriptional regulation. J. Biol. Chem. 271:28953-28959. [DOI] [PubMed] [Google Scholar]
- 45.Van Helden, J., B. André, and J. Collado-Vides. 2000. A web site for the computational analysis of yeast regulatory sequences. Yeast 16:177-187. [DOI] [PubMed] [Google Scholar]
- 46.Van Hoek, P., J. P. van Dijken, and J. T. Pronk. 2000. Regulation of fermentative capacity and levels of glycolytic enzymes in chemostat cultures of Saccharomyces cerevisiae. Enzyme Microb. Technol. 26:724-736. [DOI] [PubMed] [Google Scholar]
- 47.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1990. Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J. Gen. Microbiol. 136:395-403. [DOI] [PubMed] [Google Scholar]
- 48.Verduyn, C., E. Postma, W. A. Scheffers, and J. P. van Dijken. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501-517. [DOI] [PubMed] [Google Scholar]
- 49.Visser, W., W. A. Scheffers, W. H. Batenberg-van der Vegte, and J. P. van Dijken. 1990. Oxygen requirements of yeasts. Appl. Environ. Microbiol. 56:3785-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollbrecht, D., and F. Radler. 1973. Die Bildung höherer Alkohole bei Aminosaüremangelmutanten von Saccharomyces cerevisiae. Arch. Microbiol. 94:351-358. [PubMed] [Google Scholar]
- 51.Wach, A., A. Brachat, R. Pohlmann, and P. Philippsen. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793-1808. [DOI] [PubMed] [Google Scholar]
- 52.Welsh, F. W., W. D. Murray, and R. E. Williams. 1989. Microbiological and enzymatic production of flavor and fragrance chemicals. Crit. Rev. Biotechnol. 8:105-169. [Google Scholar]