Abstract
Lymphocyte function in vivo is dictated by multiple external cues, but the integration of different signals is not well understood. Here, we show that competition for the axis of polarization dictates functional outcomes. We investigated the effect of ligation of the immunoregulatory cell surface receptor, CD46, on lymphocyte polarity during antigen presentation and cytotoxic effector function. Ligation of CD46 on human T cells prevented recruitment of the microtubule organizing center, CD3, and perforin to the interface with the antigen-presenting cell and caused a reduction in IFN-γ production. In human NK cells, similar changes in polarity induced by CD46 ligation inhibited the recruitment of the microtubule organizing center and perforin to the interface with target cells and correlated with reduced killing. These data indicate that external signals can alter lymphocyte polarization toward antigen-presenting cells or target cells, inhibiting lymphocyte function.
Keywords: immunological synapse, measles, polar axis, T cell signaling, cytotoxicity
Lymphocyte function is influenced by multiple extracellular cues, and the response to these signals is regulated by compartmentalization of proteins into distinct domains within the cell, for instance during migration or antigen presentation (1–3). Antigen presentation involves the formation of an immunological synapse (IS) proximal to the antigen-presenting cell (APC), remodeling of the actin and tubulin cytoskeletal networks, and clustering of signaling complexes at the distal pole of an axis perpendicular to the APC (4). Intracellular compartmentalization can control the sensitivity to signal, the duration of responsiveness to signal, and the direction of effector responses such as migration, cytotoxicity, and cytokine secretion (5). The signals that trigger polarization, such as ligation of chemokine receptors or the T cell receptor (TCR), are often modulated by the ensuing polarization state (2, 5), suggesting that cell polarity provides an feedback inbuilt mechanism.
Recent reports suggest that extracellular signals compete to dictate both lymphocyte polarity and functional outcome. The intensity of signal from two target cells attached to the same T cell dictates the polarization of cytolytic granules (6). Competition between TCR and chemokine signaling determines whether a T cell adopts migratory polarization or forms an IS (7–9). To explore whether competition for polarization might provide a general means for controlling lymphocyte function, we investigated the effects of a competing polarizing signal on the response of lymphocytes to TCR or NK receptor signaling.
CD46 is a cell surface receptor for complement C3b and a number of pathogens, including measles viruses (10–12). CD46 ligation has been proposed to limit acquired immune function in response to complement and pathogens (12, 13) and facilitate the dramatic immunosuppression triggered by measles infection (14). Indeed, ligation of CD46 can mediate direct effects on T cell function, such as inducing regulatory T cells (15) and preventing cytotoxic T lymphocyte (CTL) activation (14). CD46 interacts with a polarity network in epithelial cells and T cells (16, 17), and ligation of CD46 by measles viruses induces polarization of epithelial cells (18). We show here that ligation of CD46 on lymphocytes alters cell polarity, impairing activation and effector function in response to TCR or NK cell receptor signaling. These results provide a possible mechanism for the subversion of normal immune cell signaling by pathogens that bind CD46 and suggest that conflicting polarization signals likely to occur in vivo can impact on IS formation and signaling.
Results
Lymphocytes Polarize Toward the Site of CD46 Ligation.
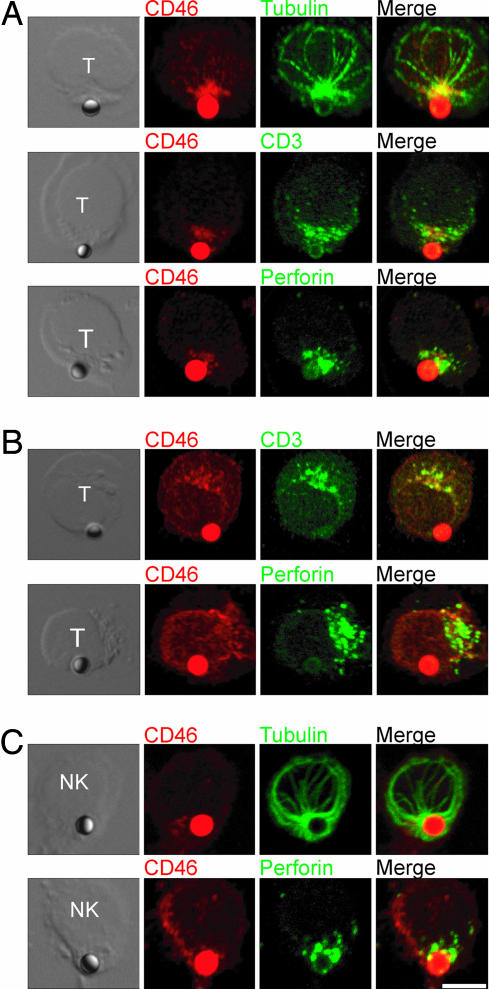
To determine whether ligation of CD46 on lymphocytes can mediate polarity changes, we incubated CTLs with anti-CD46-coated beads. We observed microtubule organizing center (MTOC) polarization to the cell–bead interface in 92% of cells conjugated to anti-CD46-coated beads (Fig. 1A). Interestingly, CD3 and perforin were also recruited to the site of CD46 ligation (Fig. 1A; 88% and 96% respectively) but not to antitransferrin receptor (TfR)-coated beads (Fig. 1B; not scored because of the low number of conjugates formed). Cross-linking of CD46 with soluble antibodies also caused patching of the CD46 and CD3 at a single site on the T cell (unpublished data). On NK cells, both the MTOC and perforin were recruited to the site of CD46 ligation (Fig. 1C; 88% and 92% respectively). The recruitment of IS components suggests a mechanism by which coligation of naïve T cells with antibodies to CD3 and CD46 coupled to the same solid surface causes an increase in activation and IFN-γ production compared with ligation of CD3 alone (15, 19, 20) (unpublished data). These results indicate that ligation of CD46 on the surface of lymphocytes induces cell polarization.
Fig. 1.
Lymphocytes polarize toward the site of CD46 ligation. (A and B) CTL were incubated for 60 min at 37°C with beads coated with 5 μg/ml rabbit polyclonal antibodies to CD46 (A) or transferrin receptor (TfR) (B), and immunolabeled for CD46 and tubulin, CD3, or perforin. (C) NK cells were incubated with anti-CD46-coated beads as above and immunolabeled for CD46 and tubulin or perforin. Images are representative of two experiments. (Scale bar: 10 μm.)
Ligation of CD46 on Naïve T Cells Alters Polarity and Signaling in Response to TCR Stimulation.
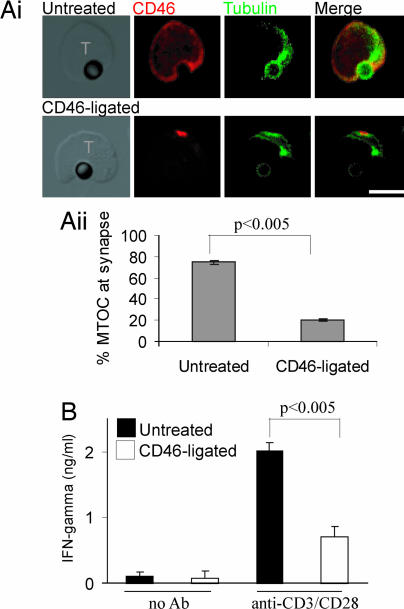
In contrast to the costimulatory effect of CD3 and CD46 coligation, ligation of CD46 separately from CD3 inhibits TCR signaling (14). To determine whether this difference involves polarity, we preincubated naïve CTLs with soluble anti-CD46 antibody and then incubated the CTLs with anti-CD3/CD28-coated beads as surrogate APCs. Untreated T cells recruited the MTOC to the interface with the bead as expected (Fig. 2Ai Upper). After CD46 ligation, the T cells formed conjugates with anti-CD3/CD28-coated beads, but polarization of the MTOC toward the bead was reduced from 75% to 20% (Fig. 2Ai Lower; and quantitated in 2Aii). The untreated T cells produced IFN-γ upon incubation with anti-CD3/CD28-coated beads, but prior ligation of CD46 significantly reduced the amount of IFN-γ produced (Fig. 2B). These data show that CD46 ligation alters T cell polarization, inhibiting the response to TCR stimulation.
Fig. 2.
Ligation of CD46 on naïve T cells alters polarization and signaling in response to TCR stimulation. (Ai) Naïve CTL (untreated or CD46-ligated) were incubated with beads coated with 5 μg/ml mAb to CD3 and CD28 and immunolabeled for CD46 and tubulin. (Scale bar: 10 μm.) (Aii) Quantitation of MTOC polarization to the bead interface. Mean ± SD is shown (50 conjugates scored per sample in triplicate). (B) Naïve CTL (untreated or CD46-ligated) were incubated with beads (antibody coated or not) for 16 h, and the supernatant was assayed for IFN-γ. Mean ± SD of triplicate samples is shown and is representative of three experiments using cells from different donors.
Ligation of CD46 on Activated T Cells Alters Polarity and IFN-γ Production Triggered by APC.
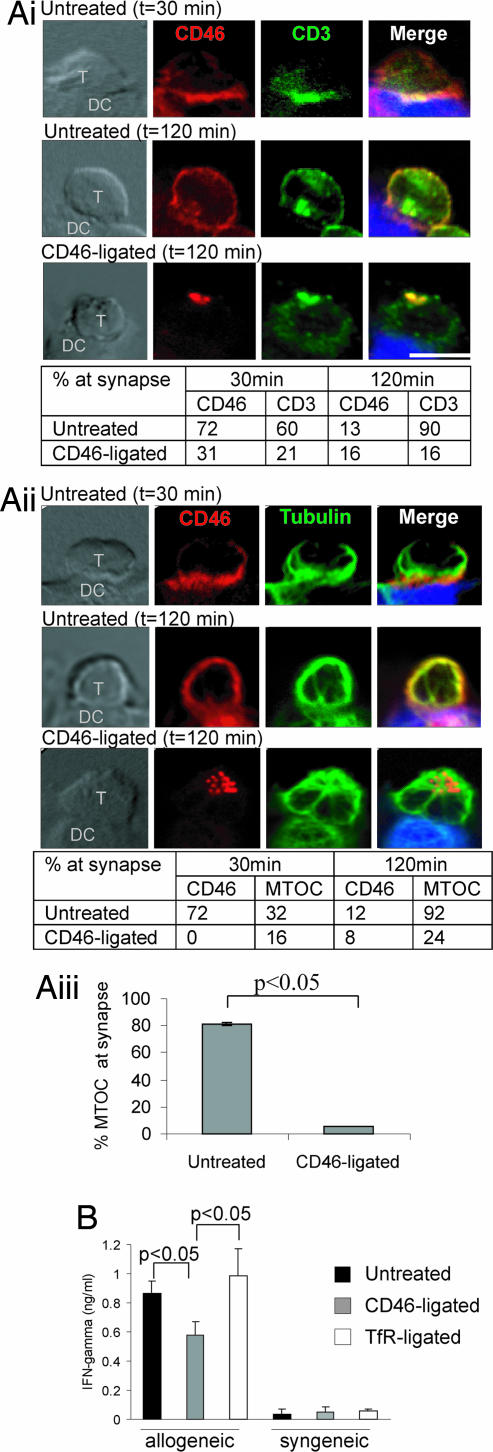
We next tested whether CD46 ligation impaired antigen presentation by professional APCs. Activated T cells formed a canonical IS upon interaction with allogeneic dendritic cells (DC), as characterized by the “bulls eye” arrangement of PKCθ surrounded by LFA1 (Fig. 6, which is published as supporting information on the PNAS web site) (4). CD46, CD3, and the MTOC all polarized to the interface with the DC at 30 min, and CD46 moved away from the interface by 120 min (Fig. 3Ai and Aii). Antibody-mediated ligation of CD46 on the T cells for 30 min before incubation with the DC did not affect the number of conjugates, but it caused a capping of CD46 to a site distal to the interface with the DC (Fig. 3 Ai and Aii Lower). Strikingly, CD3 recruitment to the interface with the DC at 120 min was reduced by CD46 ligation (Fig. 3Ai). Similarly, ligation of CD46 reduced recruitment of the MTOC to the interface with the APC (Fig. 3Aii; similar results were obtained with triplicate samples at 90 min in Fig. 3Aiii). Interestingly, both the CD46 cap and the MTOC and CD3 were predominantly located directly opposite the APC interface, suggesting the possibility that they were actively recruited to the distal pole of the T cell. Thus, ligation of CD46 prevented normal polarization of the T cell toward an APC and, indeed, prevented IS formation, by most definitions (5).
Fig. 3.
Ligation of CD46 on activated T cells reduces polarization toward the APC and IFN-γ production. (A) Activated T cells (untreated or CD46-ligated) were incubated with allogeneic DC and cell conjugates immunolabeled for CD46 and CD3 (Ai), and CD46 and tubulin (Aii). Quantitation of polarization at the APC interface is tabulated below. Images are representative of three experiments and quantitation is representative of two experiments (n = 25). (Scale bar: 10 μm.) (Aiii) Quantitation of MTOC polarization to the interface at 90 min. Mean ± SD is shown (20 conjugates scored per sample in triplicate). (B) Activated T cells (untreated or CD46-ligated) were incubated with allogeneic or syngeneic DC for 16 h, and the supernatant was assayed for IFN-γ. Mean ± SD of triplicate samples are shown and are representative of two experiments using cells from different donor sets.
To determine whether the reduced recruitment of MTOC and CD3 to the interface impaired TCR signaling and effector function, we measured IFN-γ production. T cells produced IFN-γ in response to allogeneic but not syngeneic DC, and IFN-γ production was significantly reduced by CD46, but not TfR, ligation (Fig. 3B), indicating that T cell activation in response to DC stimulation was impaired.
Ligation of CD46 on NK Cells Reduces Polarization Toward the Target Cell and Reduces Target Killing.
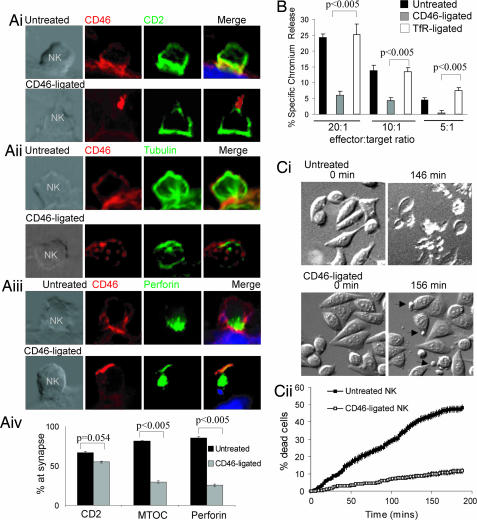
We next investigated whether CD46 ligation could affect lymphocyte cytotoxic effector function, which requires polarization of granules to the cell–target interface (21). Alloreactive T cells did not provide a sensitive readout for killing, so we investigated the effect of CD46 ligation on NK cell polarity during NK–HeLa interactions. In untreated NK cells, canonical IS formation was observed (22), with polarization of CD2, MTOC, and perforin to the interface between the NK and target cell at 15 min (Fig. 4Ai–Aiii Upper). CD46 also localized to the junction between the NK and target cell (Fig. 4Ai–Aiii Upper). In contrast, ligated CD46 capped at a point distal to the target, similar to the T cell–DC conjugates (Fig. 4 Ai–iii, Lower). Polarization of CD2 to the interface between the NK and target cell did not change significantly (from 67% to 55%), but translocation of the MTOC and perforin to the interface was reduced from 82% to 29% and 85% to 25%, respectively (Fig. 4 Ai–Aiii Lower; and quantified in Fig. 4Aiv). This change in NK polarity after CD46 ligation correlated with dramatically reduced killing of HeLa cells (25% to 6% at a 20:1 ratio; Fig. 4B). CD46-ligated NK cells still associated with the target cells (indicated by the arrows, Fig. 4Ci), but cell death was reduced from 50% to 8%, as assessed by rounding, blebbing, and detachment (Fig. 4Cii; and see Movies 1 and 2, which are published as supporting information on the PNAS web site). These data indicate that ligation of CD46 on NK cells does not affect interaction with target cells and recruitment of early scanning molecules (such as CD2) but does prevent the polarization of the MTOC and perforin to the interface with the target and significantly reduces cytotoxicity.
Fig. 4.
Ligation of CD46 on NK cells affects polarization toward the target cell and reduces target killing. (A) NK cells (either untreated or CD46-ligated) were incubated with HeLa target cells, and cell conjugates were immunolabeled for CD46 and CD2(i), CD46 and tubulin (ii), or CD46 and perforin (iii). (Scale bar: 10 μm.) (iv) Quantitation of polarization at the cell-target interface. Mean ± SD of triplicate samples is shown (n = 50). (B) The 51Cr-labeled HeLa cells were cocultured with untreated or CD46-ligated peripheral blood mononuclear cells (enriched for NK cells), and supernatants were assayed for 51Cr release. Mean ± SD of triplicate samples is shown and is representative of three experiments using cells from three donors. (C) Sequential images of NK–target cell interactions taken every 2 min over 3 h (see Movies 1 and 2). (Ci) DIC images taken at 0, 146 (untreated), and 156 (CD46-ligated) min after addition of NK cells. (Cii) Target cell death was calculated for each frame, and data are presented as the average percentage death ± SEM (n = 8 fields of ≈100 target cells per field).
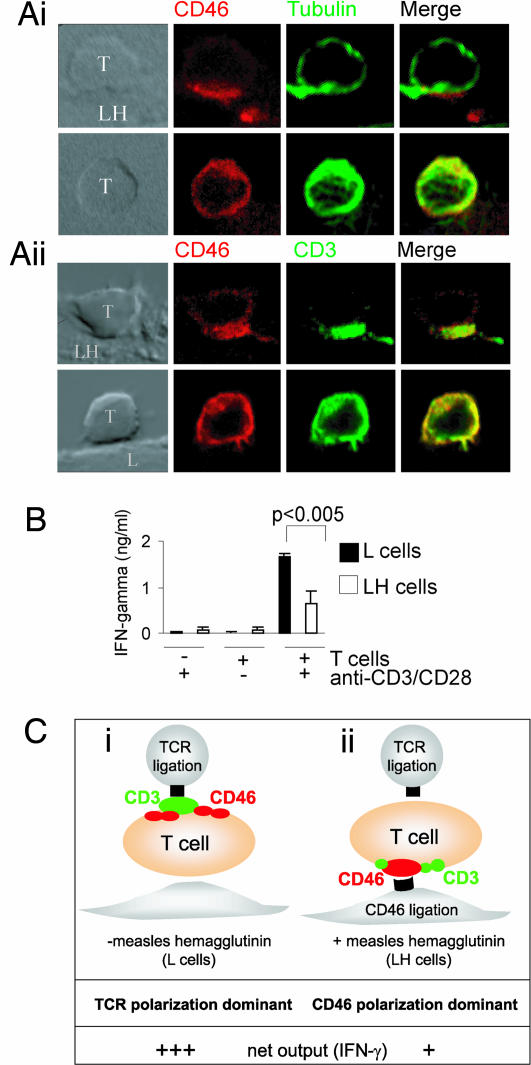
Ligation of CD46 by Bystander Cells Expressing Measles Hemagglutinin Alters T Cell Polarity and Signaling.
During measles infection, bystander cells transmit the virus through cell–cell contact, with only small amounts of free virus in circulation (23). Furthermore, immunosuppression is mediated by cell surface contact with virus rather than infection of immune cells per se (14, 24), suggesting that the effects of measles on immune cell function can be mimicked by using a cell line expressing measles hemagglutinin on its surface (LH cells) to ligate CD46 on T cells. We therefore assessed the effects of LH cells on T cell polarization. In conjugates formed between LH and T cells, CD46 was tightly capped at the point of contact, with the MTOC polarized to the site of contact in 92% of cells (Fig. 5Ai Upper) and CD3 colocalized with CD46 in 70% of cells (Fig. 5Aii Upper). MTOC recruitment depends on CD46 binding, because it was abrogated by blocking antibody or soluble CD46 extracellular domain (Fig. 7, which is published as supporting information on the PNAS web site). In control conjugates between T cells and L cells, both CD3 and CD46 were spread throughout the T cell (Fig. 5Ai Lower), and the MTOC did not translocate to the site of contact (Fig. 5Aii Lower). Addition of anti-CD3/CD28-coated beads to T cells incubated on L cells induced high levels of IFN-γ production (Fig. 5B). In contrast, T cells cultured in the presence of LH cells produced significantly less IFN-γ (Fig. 5B). These data demonstrate that bystander cells expressing measles hemagglutinin ligate CD46 on T cells and alter their polarity and activation in response to TCR signaling.
Fig. 5.
Ligation of CD46 on naïve CTL by bystander cells expressing measles hemagglutinin affects T cell function in response to TCR stimulation. (A) Naïve CTL were incubated with L or LH (L cells expressing measles hemagglutinin) cells for 60 min, and cell conjugates were immunolabeled for CD46 and tubulin or CD3. Images are representative of two experiments. (Scale bar: 10 μm.) (B) Naïve CTL were incubated with L or LH cells for 60 min, beads were added for 90 min (antibody coated or not), and the supernatant was assayed for IFN-γ. Mean ± SD of triplicate samples is shown and are representative of three experiments using cells from three donors. (C) Diagrammatic representation of effects of polarity on TCR signaling. (i) Represents canonical polarization toward the site of TCR ligation, (ii) Represents polarization toward the site of CD46 ligation by LH cells, which reduces CD3 polarization to the TCR and decreases IFN-γ production.
Discussion
We show that signals compete to determine the axis of polarization in T cells, influencing the response of T cells to subsequent signals. Specifically, by establishing polarization toward the immunoregulatory surface protein CD46, the polarization of T cells toward cognate antigen is abrogated, and effector function is inhibited. Competition for the axis of polarity therefore “tunes” a T cell toward particular signals, providing a molecular mechanism by which T cells can integrate multiple inputs in vivo. The correlation between altered polarity and reduced T function observed here is unlikely to reflect reduced CD3 at the site of antigen presentation, because only 1% of total CD3 is required for optimal signaling (25), and, indeed, the concentration of molecules at the IS is not thought to promote signaling (5, 26–28). Displacement of the MTOC, generally associated with control of cytoskeletal and vesicular trafficking, is a likely cause of reduced TCR signaling, but other events such as reorganization of the polarity network, cocapping of multiple receptors, or changes in lipid-raft trafficking might also play a role. However, our data suggest that receptor capping is not a general mechanism, because TfR capping (ref. 29 and Fig. 8A, which is published as supporting information on the PNAS web site) does not alter polarity (B) or TCR signaling (Figs. 3 and 4). Ligation of other receptors such as CD2 and LFA-1 or induction of a virological synapse by retroviral infection can trigger MTOC recruitment (30), suggesting the possibility that they might also divert polarity; however, ligation of the costimulatory molecule, CD28, does not alter polarization (Fig. 8C). Further investigation is needed to determine whether diversion of polarity is a broadly used mechanism by which signaling is regulated as well as to elucidate the mechanisms involved and the factors (such as strength and timing of signaling and duration of polarity changes) that affect the hierarchy of control of polarization.
Our observations provide a potential mechanism for the immunoregulatory function of CD46, which is proposed to occur in response to both pathogens that bind CD46 and complement-fixed cells (10, 11, 13, 31) and might explain conflicting reports on CD46 signaling in T cells. In examples where CD46 ligation exerted a positive effect on T cells, CD46 and CD3 were coligated on the same solid surface (15, 20). In contrast, inhibitory effects of CD46 were triggered when CD46 was ligated by soluble antibody, dimerized complement C3b, or measles hemagglutinin (viral or cell-bound) before TCR stimulation (by either antibody or cognate MHC peptide presentation) (14). Our results demonstrate that the initial ligation of CD46 recruits the axis of polarity away from the site of antigen presentation (Fig. 5C), whereas coligation of CD46 and CD3 on the same solid surface might enhance rather than disrupt the axis of polarity. Thus, the costimulatory or suppressive function of CD46 might be determined by whether infection or complement activation is of bystander cells, such as endothelial cells, or of APCs (12, 13).
In summary, we demonstrate that ligation of CD46 subverts cell polarization in response to antigen presentation or target cells and impairs T or NK cell function. These observations, combined with earlier examples in which signals compete to dictate polarity (6–9), suggest a new model for the integration of T cell signaling. In this model, competition between differently localized signals, which generate alternative axes of polarity, may determine the responsiveness of the T cell to subsequent spatially localized signals. Such a model would have important consequences in vivo, where lymphocytes are likely to be exposed to multiple signals simultaneously.
Materials and Methods
Reagents.
Antibodies were mouse anti-human CD3, CD28, CD4, CD8, CD1a, CD14, CD11a, CD56, perforin (δG9), and isotype-matched controls (BD Biosciences, San Diego, CA), Alexa Fluor secondary antibodies (Molecular Probes, Eugene, OR), and rabbit polyclonal to TfR and to CD46 (18). CD8+ T cell and CD56+ NK cell isolation kits were from Miltenyi Biotec (Auburn, CA).
Cells.
Peripheral blood mononuclear cells were isolated from heparinized whole blood by standard density gradient (32). Lymphocyte populations were isolated from nonadherent cells by using magnetic cell sorting (MACS) negative (CD8+) or -positive [CD56+(NK)] selection (>95% purity) and cultured in GIBCO RPMI medium 1640 with glutamine (GIBCO-BRL, Carlsbad, CA), 5% FCS/5% human AB serum and 50 IU/ml rIL-2 (Chiron, Emeryville, CA). Adherent cells (monocytes) were cultured in GM-CSF (50 ng/ml) and IL-4 (100 units/ml) (Chemicon, Temecula, CA) for 7 days to generate CD1a+CD14− DC. Human allogeneic T cells were generated by incubating PBLs from Donor B with irradiated (2,000 rad) PBL from MHC-disparate donor A for 10 days. Allogeneic T cells were generally 60% CD4+ and 40% CD8+. The murine fibroblast cell line L929, parental (L cells) or transfected with measles hemagglutinin (LH cells) (33), and HeLa cells were cultured in RPMI medium 1640 with 10% FCS.
Conjugation and Functional Responses.
Carboxyfluorescein diacetate (CFSE)-labeled DC (2.5 × 104 per well) (Donor A) were adhered overnight onto eight-well chamber slides (Nalge Nunc, Naperville, IL). T cells (Donor B) were overlaid (105 per well) for the indicated times (0 represents the time of cell addition), and cell conjugates were fixed, permeabilized, and immunolabeled (16). Purified NK cells were overlaid (5.0 × 104 per well) onto adherent HeLa cells (104 per well) for 15 min and processed as above. Then, 2 × 105 antibody-coated polystyrene beads (3.2 μm; Polysciences, Warrington, PA) were incubated with 5 × 104 cells for 2 h, adhered to glass slides by centrifugation (7 × g for 10 min) and processed as above. For Ab ligation, cells were incubated with 4–10 μg/ml CD46 (1840), CD28, or TfR antibody for 30 min at 37°C. Confocal images were acquired and processed by using a BX61 microscope (Olympus, Melville, NY) with Olympus Fluorview FV1000 laser-scanning confocal and software (Olympus, Japan). Live-cell imaging was carried out as described (16). Movies were compiled from individual images and play at four frames per sec. Scoring of polarization was performed blind on at least 50 conjugates (25 for allogeneic conjugates), and molecules were considered polarized toward the target cell if >50% of fluorescence was detected at the interface. The 51Cr-labeled cells (2 × 104) were incubated with peripheral blood mononuclear cells enriched for NK cells (50%) for 4 h at 37°C and 51Cr (cpm) released into the supernatant was detected by using a Wallac Wizard 1470 automatic γ counter (PerkinElmer Life Sciences, Boston, MA). Human IFN-γ was measured by ELISA (Pierce, Rockford, IL). P values were determined by two-tailed Student's t test.
Supplementary Material
Acknowledgments
We thank C. Clarke, P. Humbert, K. Poetter, and M. Smyth for critical reading of the manuscript; Y. Hayakawa for helpful discussion; Denis Gerlier (Institut Fédératif Laennec, Lyon, France) for LH cells and MCI20.6 antibody; and Bruce Loveland (Austin Research Institute, Victoria, Australia) for CD46 antibody and recombinant CD46. This work was supported by National Health and Medical Research Council (NHMRC) Grants 251619, 350461, and 400043; Australian Research Council Grant DP0451224; a Wellcome Fellowship (to S.M.R.); an R. D. Wright Fellowship (to N.J.W.); and an NHMRC Senior Principal Research Fellowship (to J.A.T.).
Abbreviations
- APC
antigen-presenting cell
- CTL
cytotoxic T lymphocyte
- DC
dendritic cell
- IS
immunological synapse
- LH
L cells expressing measles hemagglutinin
- MTOC
microtubule organizing center
- TCR
T cell receptor
- TfR
antitransferrin receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Russell S, Oliaro J. Immunol Cell Biol. 2006;84:107–113. doi: 10.1111/j.1440-1711.2005.01415.x. [DOI] [PubMed] [Google Scholar]
- 2.Manes S, Gomez-Mouton C, Lacalle RA, Jimenez-Baranda S, Mira E, Martinez-AC Semin Immunol. 2005;17:77–86. doi: 10.1016/j.smim.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Cullinan P, Sperling AI, Burkhardt JK. Immunol Rev. 2002;189:111–122. doi: 10.1034/j.1600-065x.2002.18910.x. [DOI] [PubMed] [Google Scholar]
- 4.van der Merwe AP, Davis SJ, Shaw AS, Dustin ML. Semin Immunol. 2000;12:5–21. doi: 10.1006/smim.2000.0203. [DOI] [PubMed] [Google Scholar]
- 5.Dustin ML. Semin Immunol. 2005;17:400–410. doi: 10.1016/j.smim.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Depoil D, Zaru R, Guiraud M, Chauveau A, Harriague J, Bismuth G, Utzny C, Muller S, Valitutti S. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Dustin ML, Bromley SK, Kan Z, Peterson DA, Unanue ER. Proc Natl Acad Sci USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez-A C, Manes S, Viola A. Nat Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 9.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 10.Liszewski MK, Kemper C, Price JD, Atkinson JP. Spring Semin Immunopathol. 2005;27:345–358. doi: 10.1007/s00281-005-0002-3. [DOI] [PubMed] [Google Scholar]
- 11.Russell S. Tissue Antigens. 2004;64:111–118. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 12.Carroll MC. Nat Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 13.Longhi MP, Harris CL, Morgan BP, Gallimore A. Trends Immunol. 2006;27:102–108. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Nat Immunol. 2002;3:659–666. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 15.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Nature. 2003;421:388–392. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 16.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, et al. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Ludford-Menting MJ, Thomas SJ, Crimeen B, Harris LJ, Loveland BE, Bills M, Ellis S, Russell SM. J Biol Chem. 2002;277:4477–4484. doi: 10.1074/jbc.M108479200. [DOI] [PubMed] [Google Scholar]
- 18.Crimeen-Irwin B, Ellis S, Christiansen D, Ludford-Menting MJ, Milland J, Lanteri M, Loveland BE, Gerlier D, Russell SM. J Biol Chem. 2003;278:46927–46937. doi: 10.1074/jbc.M308261200. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez A, Jose Feito M, Rojo JM. Eur J Immunol. 2004;34:2439–2448. doi: 10.1002/eji.200324259. [DOI] [PubMed] [Google Scholar]
- 20.Astier A, Trescol-Biemont M-C, Azocar O, Lamouille B, Rabourdin-Combe C. J Immunol. 2000;164:6091–6095. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 21.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- 22.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. Proc Natl Acad Sci USA. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firsching R, Buchholz CJ, Schneider U, Cattaneo R, ter Meulen V, Schneider-Schaulies J. J Virol. 1999;73:5265–5273. doi: 10.1128/jvi.73.7.5265-5273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlender J, Schnorr J-J, Spielhofer P, Cathomen T, Cattaneo R, Billeter MA, ter Meulen V, Schneider-Schaulies S. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei X, Tromberg BJ, Cahalan MD. Proc Natl Acad Sci USA. 1999;96:8471–8476. doi: 10.1073/pnas.96.15.8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Keefe JP, Blaine K, Alegre M-L, Gajewski TF. Proc Natl Acad Sci USA. 2004;101:9351–9356. doi: 10.1073/pnas.0305965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K-H, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 28.Lee K-H, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, et al. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 29.Enns CA, Larrick JW, Suomalainen H, Schroder J, Sussman HH. J Cell Biol. 1983;97:579–585. doi: 10.1083/jcb.97.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnard AL, Igakura T, Tanaka Y, Taylor GP, Bangham CRM. Blood. 2005;106:988–995. doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- 31.Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Oliaro J, Dudal S, Liautard J, Andrault J-B, Liautard J-P, Lafont V. J Leukoc Biol. 2005;77:652–660. doi: 10.1189/jlb.0704433. [DOI] [PubMed] [Google Scholar]
- 33.Krantic S, Gimenez C, Rabourdin-Combe C. J Gen Virol. 1995;76:2793–2800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 34.Krantic S, Gimenez C, Rabourdin-Combe C. J Gen Virol. 1995;76(Pt 11):2793–800. doi: 10.1099/0022-1317-76-11-2793. [DOI] [PubMed] [Google Scholar]
- 35.Lanteri MB, Powell MS, Christiansen D, Li YQ, Hogarth M, Sandrin MS, McKenzie IF, Loveland BE. Transplantation. 2000;69:1128–1136. doi: 10.1097/00007890-200003270-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.