Abstract
Most dioecious plant species are believed to derive from hermaphrodite ancestors. The regulatory pathways that have been modified during evolution of the hermaphrodite ancestors and led to the emergence of dioecious species still remain unknown. Silene latifolia is a dioecious plant species harboring XY sex chromosomes. To identify the molecular mechanisms involved in female organ suppression in male flowers of S. latifolia, we looked for genes potentially involved in the establishment of floral organ and whorl boundaries. We identified homologs of Arabidopsis thaliana SHOOTMERISTEMLESS (STM) and CUP SHAPED COTYLEDON (CUC) 1 and CUC2 genes in S. latifolia. Our phylogenetic analyses suggest that we identified true orthologs for both types of genes. Detailed expression analyses showed a conserved expression pattern for these genes between S. latifolia and A. thaliana, suggesting a conserved function of the corresponding proteins. Comparative in situ hybridization experiments between male, female, and hermaphrodite individuals reveal that these genes show a male-specific pattern of expression before any morphological difference become apparent. Our results make SlSTM and SlCUC strong candidates for being involved in sex determination in S. latifolia.
Keywords: CUC gene, sex determination, STM gene
Most Angiosperm species are hermaphrodite and develop bisexual flowers. These include model species such as Arabidopsis thaliana, Antirrhinum majus, and Petunia hybrida, species from which genes involved in different steps of flower development have been identified. An extensive list of genes are known to be involved in processes such as floral meristem identity, floral organ identity, establishment of organ and whorl boundaries, organ polarity, and flower symmetry (see ref. 1 for review).
Dioecious plant species (with separate male and female individuals) represent only a few percent of the angiosperm species but are widely scattered taxonomically. A large proportion of angiosperm families have dioecious members. It is therefore likely that dioecy evolved several times independently in different plant lineages (2, 3). Because most of the dioecious species develop potentially hermaphrodite floral meristems, which subsequently differentiate into male or female flowers, it is assumed that each occurrence of dioecy must have evolved from a hermaphrodite ancestor (2, 4–6). Additionally, in each dioecious species, the sexual dimorphism results from distinct regulatory modifications of the bisexual condition. The molecular mechanisms underlying dioecy, and therefore sex determination in plants, are largely unknown.
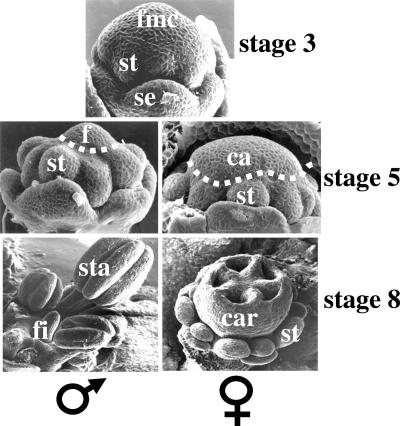
In the dioecious species Silene latifolia, as in any hermaphrodite species, four whorls of floral organs are observed in both male and female floral meristems: sepals, petals, stamens (male reproductive organs), and carpels (female reproductive organs). At an early stage, when sepal primordia are visible (stage 3; all stages are according to ref. 7), the flower meristem is similar in male and female plants (undifferentiated). As soon as all floral organ primordia are initiated (stage 5), the female territory in the center of the flower meristem is significantly smaller in male compared with female flower buds (Fig. 1). Later, in male flowers, a filament will develop in place of female organs (see Fig. 1). In female flower buds, stamens are initiated but rapidly degenerate, whereas five fused carpels (female organs) develop in the center (7).
Fig. 1.
Sexual dimorphism in S. latifolia. Shown are flower meristems observed by scanning electron microscopy. Developmental stages are as defined by Farbos et al. (7). At stage 3, two types of primordia are visible: sepal and stamen (petal primordia are initiated later and are not yet visible). The center of the flower meristem has not yet initiated carpel primordia. No difference is observed between flower meristem from male or female plant. At stage 5, all of the primordia are initiated. In flower meristem from female plants, carpel primordia appear as a wide dome. In flower meristem from male plants, the central dome is five times smaller than in the females and corresponds to the filament primordia. At stage 8, the difference between males and females is very clear. The five fused carpels in the center of the female flower are well developed, whereas the center of the male flower exhibits an undifferentiated filament. se, sepal primordia; st, stamen primordia; fmc, flower meristem center; ca, carpel primordia; f, filament primordia; sta, stamen; fi, filament; car, carpel.
In S. latifolia, the sexual phenotype is controlled by X and Y sex chromosomes. Two independent loci in the Y chromosome are responsible for sex determination (8, 9). The first locus is responsible for the early arrest of female organs in male flowers (visible at stage 5), and when it is mutated or deleted, hermaphrodite flowers develop (9). The second locus activates the development of male organs in male flowers (Fig. 1). However, the corresponding genes and the regulatory pathways involved in sex determination have yet to be identified. Because the sexual dimorphism is expressed very early during flower organogenesis, reproductive organ identity genes (the B and C functions of the ABC model; see ref. 10 for review) have been believed to play a key role in this process (11). Hardenack et al. (11) showed that in both male and female flower buds, these floral organ identity genes have the same expression pattern as that in hermaphrodite species. The authors concluded that sex determination genes must act either downstream from these organ identity genes or in a parallel pathway.
In this study, we investigated the possible mechanisms that may lead to female organ arrest in male flowers of S. latifolia. Based on data of Fig. 1 (compare the center of the flower meristem in male and female at stage 5), it is clear that there is a whorl-specific arrest in cell proliferation in the early male flower meristem. This reduced proliferation results in the formation of a filament in the center of male flowers at a later stage (Fig. 1). From these observations, we suspected a precocious arrest of the flower meristem in male individuals. We therefore decided to look for the orthologs in S. latifolia of two genes central to meristem function in A. thaliana: WUSCHEL (WUS) and SHOOTMERISTEMLESS (STM). We also looked for CUP SHAPED COTYLEDON (CUC) 1 and CUC2, which have been shown to participate in meristem homeostasis in concert with STM (12). Despite several attempts, we could not identify any WUS ortholog in S. latifolia. However, our phylogenetic reconstructions suggest that we have indeed identified orthologs of A. thaliana STM and CUC1 and CUC2 in S. latifolia. We performed in situ hybridization on young flower buds from male, female, and hermaphrodite plants. Both orthologs showed clear differences in their expression pattern between males and females or hermaphrodites, which suggests their possible involvement in the sex determination pathway in S. latifolia.
Results
Identification of STM and CUC1 and CUC2 Orthologs.
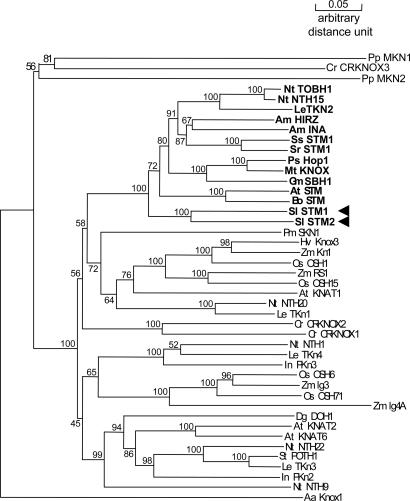
Degenerate primers (see Materials and Methods) used to amplify orthologs of STM were designed against the region spanning the MEINOX domain and the homeodomain, both of which are highly conserved among all of the STM orthologs examined to date in different species. We performed RT-PCR on S. latifolia total RNA extracted from shoot apices of germinating seedlings. Among the clones analyzed, we found two 900-bp-long STM-like cDNAs. Alignment with the available KNOX gene sequences already used by Harrison et al. (13) showed that both sequences contain all typical features of class I KNOX genes, including regions encoding the MEINOX domain, the ELK domain, and the homeodomain. The alignment was used to construct a phylogenetic tree (Fig. 2). Both cDNA sequences group with the STM clade (class I KNOX genes), and the branch is supported by a strong bootstrap value, which suggests that we indeed cloned two STM orthologs. We called these SlSTM1 and SlSTM2.
Fig. 2.
Phylogenetic tree of KNOX gene cDNA sequences. STM-like genes from dicotyls are highlighted in bold, and SlSTM1 and SlSTM2 are indicated with arrowheads. AaKnox1, Pp MKN1 and PpMKN2, and CrCRKNOX3 were included as outgroups. The numbers beside the branches represent bootstrap values based on 500 replicates.
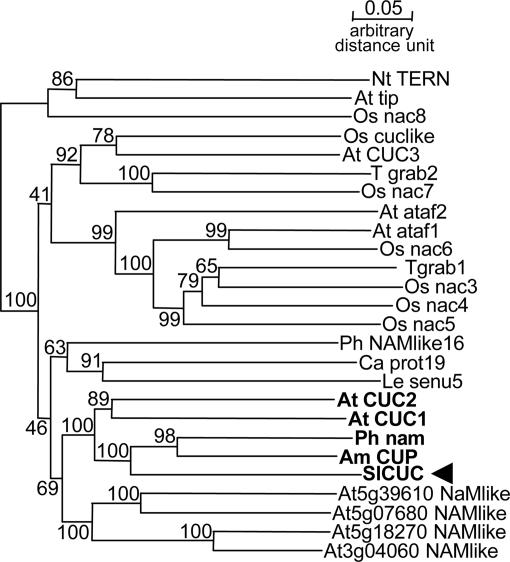
Primers used to amplify orthologs of CUC1 and CUC2 were designed by using the CODEHOP method (14) against the region spanning the conserved NAC domain and the miR164 binding site. Among the amplified sequences potentially encoding NAC domain, we identified a 500-bp-long CUC-like sequence that we aligned with NAC genes, including CUC orthologs, to construct a phylogenetic tree (Fig. 3). The clone from S. latifolia groups with NAM (NO APICAL MERISTEM) from P. hybrida, with CUP (CUPULIFORMIS) from Antirrhinum majus, and with both CUC1 and CUC2 (but not CUC3), from A. thaliana. The branch is supported by a strong bootstrap value, suggesting that we indeed cloned a CUC1 and CUC2 ortholog, which we named SlCUC.
Fig. 3.
Phylogenetic tree of NAC gene cDNA sequences. The members of the CUC clade are highlighted in bold, and the S. latifolia gene is indicated with an arrowhead. The numbers beside the branches represent bootstrap values based on 500 replicates.
RT-PCR Analysis.
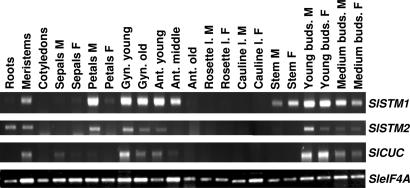
The expression patterns of SlSTM1, SlSTM2, and SlCUC were initially analyzed by RT-PCR on different plant tissues from male and female individuals. Specific primers were designed for each gene, and transcript accumulation was normalized according to eIF4A transcript levels (15). Results are presented in Fig. 4. Both SlSTM1 and SlSTM2 are expressed in shoot apical meristems (meristems), young and medium flower buds from both males and females, petals from male flowers, both young and old gynoecium, and young anthers. Some differences were observed between SlSTM1 and SlSTM2, because SlSTM1 is also expressed in middle anthers and in stems from both males and females, whereas SlSTM2 is expressed in roots. Apart from its expression in stems, SlSTM1 shows an expression pattern very close to its A. thaliana ortholog STM (16).
Fig. 4.
RT-PCR analyses of SlSTM1, SlSTM2, and SlCUC in S. latifolia tissues. The expression patterns of SlSTM1, SlSTM2, and SlCUC were investigated by RT-PCR analysis on the tissues indicated above each lane. The genes amplified are indicated on the right. SleIF4A was used as an internal reference. PCR products were analyzed on agarose gels and visualized under UV light in the presence of ethidium bromide. M, male; F, female; Gyn, gynoecium; Ant, anther; l, leaf.
SlCUC is expressed in shoot apical meristems and in flower buds from both males and females (Fig. 4). It is also expressed in the gynoecium and in young anther. This expression pattern is very similar to that of CUC1 and CUC2 from A. thaliana (17, 18).
In Situ Hybridization Analyses.
To investigate in more detail the expression pattern of SlSTM1, SlSTM2, and SlCUC and evaluate their potential implication in sex determination, we performed in situ hybridization experiments on young flower meristems from male, female, and hermaphrodite (bsx11 mutant) individuals. The bsx11 hermaphrodite mutant lost the portion of the Y chromosome responsible for the arrest of carpel development (9) and therefore develops a functional gynoecium. We have included this Ydeleted hermaphrodite mutant in the analysis to make sure that the differences observed between males and females were linked to the absence of carpels in males and not to pleiotropic, unrelated differences between males and females. Developmental stages of S. latifolia flower meristems correspond to those described by Farbos et al. (7). At stage 2, the flower meristem is round, and no primordia are yet visible. At stage 3, the sepal primordia are initiated. During stage 4, petal and stamen primordia are successively initiated, and at stage 5, all organ primordia are formed (Fig. 1). At stage 6, floral organs start to differentiate (7).
SlSTM1 and SlSTM2.
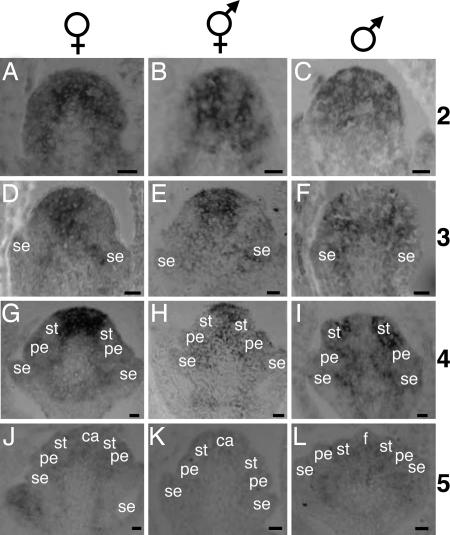
We first performed in situ hybridization using probes specific for SlSTM1 or SlSTM2. The pattern of expression was similar for the two genes (data not shown). Because a stronger signal was observed with the 900-bp SlSTM1 cDNA probe that hybridized to both types of transcripts, this probe was used for further analyses. The pattern of accumulation of both SlSTM1 and SlSTM2 transcripts is shown in Fig. 5. A strong signal was detected at stage 2 (Fig. 5 A–C) in all of the cells of the male, female, and hermaphrodite flower meristems. In flower meristems from female and hermaphrodite, from stages 3 to 5 (Fig. 5 D, E, G, H, J, and K), we observed a progressive restriction of SlSTM1 and SlSTM2 transcripts toward the inner part of the flower meristem. No signal was detected in organ primordia. When carpel primordia are initiated at stage 5, SlSTM1 and SlSTM2 transcripts were no longer detected (Fig. 5 J and K). Strikingly, no transcripts were detected in the center of the male flower meristem from stage 3 onwards (Fig. 5 F, I, and L). This absence of signal was particularly evident at stages 3 (Fig. 5F) and 4 (Fig. 5I), whereas SlSTM1 and SlSTM2 transcripts were still present in regions where future primordia would develop. Depending on the plane of the sections, some signal could also be detected around the primordia and in vascular tissues (Fig. 5 F, H, and I).
Fig. 5.
In situ hybridization with a SlSTM1 and SlSTM2 probe. Sections of S. latifolia flower buds hybridized with a SlSTM1 and SlSTM2-specific probe. The signal appears in gray or black. The sex of the individuals is indicated above each column, and the stages of development are indicated on the right of each row. se, sepal primordia; pe, petal primordia; st, stamen primordia; ca, carpel primordia; f, filament primordia. (Scale bars: 20 μm.)
SlCUC.
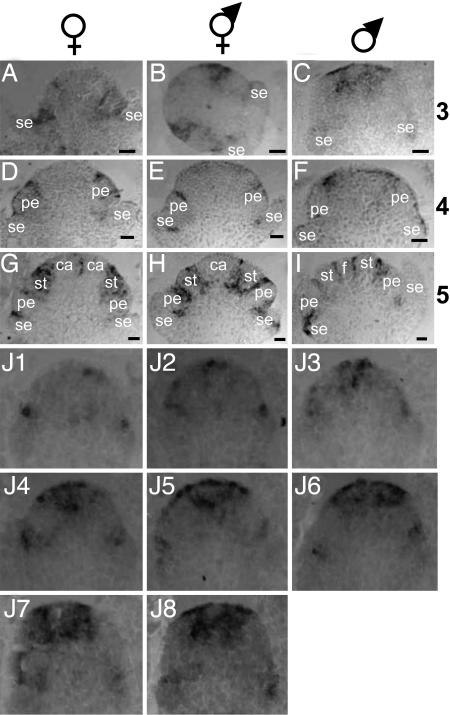
The pattern of accumulation of the SlCUC transcripts is shown in Fig. 6. SlCUC was detected at the boundaries between whorls before each type of primordia became visible from stage 3 to stage 5. This sequence was observed in flower meristems of male, female, and hermaphrodite. An enlarged signal could be observed in some sections (Fig. 6 B and G) and probably corresponds to the future interprimordia boundary within the same whorl because of the plane of the section. Remarkably, at stage 3 and in male meristems only, SlCUC was detected in a wide region in the center of the meristem (Fig. 6C). This expression was transient; it was not observed at later stages. Serial sections of a male flower meristem at stage 3 are shown in Fig. 6J (sections 1–8 from the periphery toward the center). Interestingly, the very inner zone of the region expressing SlCUC was devoid of signal and may correspond to the area where the filament will develop.
Fig. 6.
In situ hybridization with SlCUC probe. Sections of S. latifolia flower buds hybridized with a SlCUC-specific probe. The signal appears in gray or black. The sex of the individuals is indicated above each column, and the stages of development are indicated on the right of each line. se, sepal primordia; pe, petal primordia; st, stamen primordia; ca, carpel primordia; f, filament. (Scale bars: 20 μm.) (J) Serial sections (J1–J8 from outside to inside) of a male flower meristem at stage 3.
SlSTM1, SlSTM2, and SlCUC Are Not Sex-Linked.
To determine whether SlSTM1 and SlSTM2 and SlCUC were located on sex chromosomes (and more precisely on the Y chromosome), we performed Southern blot experiments using these genes as probes against genomic DNA from a segregating family of male and female individuals. We found no evidence of sex linkage for these genes (Fig. 7, which is published as supporting information on the PNAS web site).
Discussion
Sexual dimorphism in the dioecious species S. latifolia occurs very early during flower development. As soon as all of the floral primordia are initiated, the central zone of the floral meristem, which corresponds to the female territory, is five times smaller in males than in females, revealing a whorl-specific restriction of cell proliferation (7). Genes involved in meristem function are essential to determine the patterns of proliferation that lead to the correct position of organ primordia and boundaries (19, 20). Such genes have been extensively characterized in A. thaliana, and we identified the corresponding genes in S. latifolia to investigate their possible involvement in sex determination. In this study, we report the cloning of STM and CUC1 and CUC2 orthologs in the dioecious species S. latifolia and show that they are likely involved in the molecular mechanisms underlying sex determination in S. latifolia.
Conserved Roles of STM and CUC Orthologs in Eudicots.
In A. thaliana, STM is required not only for the establishment of the shoot meristem but also for the maintenance of undifferentiated cells in the shoot meristem and for proper proliferation of cells in the floral meristem (16, 21–23). It is expressed in the shoot apical meristem, the inflorescence meristem, and the floral meristem. The transcript is also detected in vascular tissues and in boundaries between floral whorls (16). STM transcripts are down-regulated in incipient floral primordia (16). Our results show a similar pattern of expression of SlSTM1 and SlSTM2 in flower meristems of S. latifolia, although the expression pattern is not strictly identical between SlSTM1 and SlSTM2 (see RT-PCR results in Fig. 3). Similar patterns of expression have also been observed for HIRZINA (HIRZ) and INVAGINATA (INA), two STM orthologs in Antirrhinum majus (24). More recently, STM orthologs have been characterized in two Papaveraceae species, Chelidonium majus and Eschscholzia californica, and have been shown to be down-regulated in organ primordia, including floral primordia (25). It is therefore likely that in S. latifolia and Antirrhinum majus species, the two paralogs are at least partially redundant. The presence of two STM paralog genes has also been noted in the genus Streptocarpus (13). Our phylogenetic analyses suggest that the duplication events in S. latifolia and Antirrhinum species are recent and independent in the two lineages. There are not enough data to draw any conclusions on the origin of the duplication events in Streptocarpus.
In A. thaliana, three CUC genes are present. All of them and their orthologs, the NO APICAL MERISTEM (NAM) gene in P. hybrida (26) and the CUPULIFORMIS (CUP) gene in Antirrhinum majus (27), are involved in the establishment and maintenance of organ boundaries in the shoot apical meristem, the inflorescence meristem, and the floral meristem (17, 28, 29). These genes are expressed in one or two rows of cells that correspond to the boundaries around each organ primordia (18). Our in situ data support an involvement of SlCUC in the control of organ boundaries in S. latifolia.
All of the above-mentioned results suggest that regulatory mechanisms controlling meristem homeostasis are presumably conserved among eudicots. SlSTM1 and SlSTM2 and SlCUC are therefore likely to control apical meristem functions in S. latifolia in a way similar to how STM and CUC1 and CUC2 control functions in A. thaliana.
Identification of a Candidate Regulatory Pathway for Sex Determination.
In the model plant A. thaliana, CUC1 and CUC2 are required for STM activation during shoot apical meristem (SAM) establishment in the embryo (17, 30). However, STM is required for proper spatial expression of CUC2: in the stm-1 mutant, CUC2 is misexpressed, because it is detected in the central region between the cotyledon primordia (where STM transcripts are normally expressed), whereas in the presence of STM, CUC2 is restricted to the boundaries between meristem and primordia (30). STM also promotes CUC1 activity (31). All these data support the existence of a regulatory feedback loop between STM and CUC1 and CUC2 in apical meristems.
In this paper, we show a clear difference in the expression pattern of SlSTM1 and SlSTM2 and SlCUC in males compared with females and hermaphrodites. SlCUC is expressed in the central region of the male floral meristem at stage 3, concomitantly with the disappearance of SlSTM1 and SlSTM2 from the same region. Interestingly, CUC genes have been shown to be associated with the absence of cell proliferation, particularly in whorl boundaries (18). In addition, Weir et al. (27) have shown a direct interaction between CUPULIFORMIS (CUP) (a CUC ortholog in Antirrhinum majus) and a TCP transcription factor known to be associated with inhibition of proliferation. In S. latifolia, such an inhibition of proliferation could result in the reduced number of cells observed in the center of male flower meristems.
The disappearance of SlSTM1and SlSTM2in male buds from stage 3 onwards presumably reflects an early arrest in meristem function and could therefore be the cause of the lack of cell divisions observed. It is likely that the central region of the meristem loses its meristematic identity and therefore behaves as it does in a weak stm mutant, where meristematic cells are rapidly consumed and differentiated. The filament would then be the product of a precocious determinacy of the male floral meristem. The formation of a filament associated with a defect in meristem function has indeed already been reported. In hairy meristem (ham) flower meristems of P. hybrida, PhWUS and PhSTM disappear precociously, and the meristematic cells are consumed to form a filament instead of carpels (32). In the A. thaliana ago1-11 stm-2 double mutant, flowers are replaced by filaments (33). Consistent with an early loss of meristematic activity in male flower meristem of S. latifolia, Matsunaga et al. (34) observed a strong reduction in cell division in the central region of male flower meristem. The reduced proliferative activity, however, was observed at stage 5 and could be a direct consequence of the lack of SlSTM expression at stage 3, as described here.
Relationship Between SlSTM1 and SlSTM2 and SlCUC in Flower Meristems.
The fact that SlSTM1 and SlSTM2 and SlCUC show a misexpression pattern in the same place and at the same time (in male flower meristems at stage 3, compared with females and hermaphrodites) could indicate that a regulatory feedback loop also exists between these genes in the dioecious species S. latifolia. Because the expression pattern of SlCUC in the male flower meristem resembles the expression pattern of CUC2 in the stm-1 mutant (31), it is tempting to correlate the lack of SlSTM1 and SlSTM2 at stage 3 (in the center of the male flower meristem) with the concomitant expansion of SlCUC. We therefore propose that in male flower meristems of S. latifolia, the disappearance of SlSTM1 and SlSTM2 would then lead to the expansion of SlCUC in the center of the meristem. This expansion being only transient, it is likely that a mechanism controlling this expansion would subsequently be turned on. Interestingly, Laufs et al. (20) have proposed that the microRNA miR164 constrains the expansion of the boundary domain by degrading CUC1 and CUC2 transcripts. The miR164 target site is present in SlCUC because it has been used as a primer to amplify the gene. In addition, because of the good conservation of microRNA during plant evolution (35), it is possible that SlCUC expansion is controlled by miR164 in flower meristems from S. latifolia.
In summary, the concomitant extinction of SlSTM1 and SlSTM2 and expression of SlCUC in the center of the male flower meristem at stage 3 are completely consistent with the arrest of proliferation observed in later stages. Interestingly, this male-specific pattern of expression is observed before any morphological differences between males and females and could therefore well be responsible for it. Consequently, the Y-linked locus responsible for gynoecium arrest in male flowers from S. latifolia presumably acts upstream from the regulatory pathway involving SlSTM1, SlSTM2, and SlCUC. This regulatory pathway could be subjected to epigenetic regulation, because treatment of male plants with 5-azacytidin, a demethylating agent, has been shown to induce hermaphrodite flower development (36). However, the number of carpels (from 0.5 to 3) in hermaphrodite mutants obtained by deletions in the Y chromosome has been shown to increase at each cycle of self-fertilization, showing an incomplete penetrance of the phenotype (9). These data suggest an epigenetic regulation of carpel suppression and a role for methylation in particular.
In conclusion, we have identified a regulatory pathway that is a strong candidate for being involved in sex determination in S. latifolia. Our results open perspectives for future research in the field.
Materials and Methods
Plant Material.
To obtain S. latifolia seedlings, seeds were surface-sterilized and germinated for 6 days in sterile conditions. S. latifolia plants were grown in a greenhouse. The stages of the flower buds were assessed according to ref. 7: young buds were less than 2.5 mm long, medium buds were between 2.5 and 10 mm long, and open flowers were longer than 10 mm. Young gynoecia were collected from young flower buds and old gynoecia from open flowers. Young anthers were collected from young flower buds, middle anthers from medium flower buds, and old anthers from open flowers. S. latifolia hermaphrodite mutants with the Y chromosome deleted have been produced in our group by x-irradiation of pollen grains and have been described in ref. 9. The bsx11 mutant used in this study is one of them.
Ortholog Identification.
Total RNA was extracted from S. latifolia shoot apices from 6-day-old seedlings by using TRIzol Reagent according to the manufacturer's instructions (Invitrogen, Cergy Pontoise, France). The oligo(dT) primer T11VN was used to synthesize the cDNA. To amplify STM orthologs, RT-PCR was done by using STM primers AAGATYATGGCTCATCCTCACTA (forward) and TCCATBACHACAAACTGCAT (reverse). The PCR conditions were as follows: 3 min at 94°C, followed by 20 cycles of 20 sec at 94°C; 1 min at 40°C (minus 1°C at each cycle) and 1 min at 72°C, followed by 15 cycles of 15 sec at 94°C; 1 min at 20°C and 1 min at 72°C, followed by a final extension of 7 min at 72°C. To amplify CUC orthologs, RT-PCR was performed by using CUC primers TTGGGAACTTCCTTGGAAGGCTCCRATGGGNGARAA (forward) and TYGGAGAARCAGGDCACGT (reverse). The PCRs were performed in two steps. Step one, with the CUC-reverse primer only, was performed as follows: 3 min at 94°C followed by 18 cycles of 20 sec at 94°C, 1 min at 65°C (minus 0.3°C at each cycle), and 1 min at 72°C. Step two, with both primers, was performed as follows: 18 cycles of 15 sec at 94°C, 1 min at 55°C (minus 0.5°C at each cycle), and 1 min at 72°C, followed by 18 cycles of 15 sec at 94°C, 1 min at 55°C, and 1 min at 72°C, followed by a final extension of 7 min at 72°C. PCR products were cloned into the pGEM-T easy vector (Promega, Madison, WI). The complete 3′ sequences of SlSTM1 and SlSTM2 were obtained by using STM-forward in a 3′ RACE-PCR experiment. Nucleotide sequences were determined by using the ABI Big Dye Terminator V1.1 DNA sequencing kit on a 3100 DNA Sequencer (Applied Biosystems, Foster City, CA).
Phylogenetic Analysis.
The sequences of the published orthologs of STM and CUC were obtained from GenBank. Nucleic acid sequences were aligned with the ClustalW program (37) and refined by hand by using the graphical multiple-sequence alignment editor SeaView (38). Ambiguous positions in the alignment were excluded from the analysis. AaKNOX1, MKN1, and CrKNOX3 were used as outgroups to root the KNOX tree, as described in ref. 13. The NAC tree was rooted by using NtTERN and AtTIP. We used the graphical color interface Phylo_win (38) to construct and bootstrap phylogenetic trees, using the neighbor-joining method, with 500 bootstrap replicates.
Expression Analyses.
RT-PCRs using specific primers for SlSTM1, SlSTM2, SlCUC, and for a constitutively expressed S. latifolia gene [eIF4A (15)] were performed according to the instructions in ref. 15. In situ hybridization with SlSTM1 and SlCUC antisense strand riboprobes was performed as described in ref. 39. In situ hybridization images were captured under bright-field illumination with a Nikon Optiphot-2 (Champigny-sur-Marne, France) Axiovert 125 inverted microscope (Zeiss, Thornwood, NY).
Supplementary Material
Acknowledgments
We thank Frédérique Rozier for assistance with in situ hybridization and Christophe Tréhin, Charlie Scutt, Sophie Jasinski, Thierry Gaude, and Yvon Jaillais for helpful discussion and critical reading of the manuscript. J.Z. was funded by the North American Treaty Organization and Ecole Normale Supérieure de Lyon. This work was done at IFR128 BioSciences Lyon-Gerland.
Footnotes
References
- 1.Smyth DR. Plant Cell. 2005;17:330–341. doi: 10.1105/tpc.104.030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainsworth C, Parker J, Buchanan-Wollaston V. Curr Top Dev Biol. 1998;38:167–223. doi: 10.1016/s0070-2153(08)60247-1. [DOI] [PubMed] [Google Scholar]
- 3.Charlesworth D, Guttman DS. In: Sex Determination in Plants. Ainsworth C, editor. Oxford, UK: Bios Scientific; 1999. pp. 25–49. [Google Scholar]
- 4.Charlesworth B. Science. 1991;251:1030–1032. doi: 10.1126/science.1998119. [DOI] [PubMed] [Google Scholar]
- 5.Darwin CR, Sessions A. The Different Forms of Flowers on Plants of the Same Species. London: Murray; 1877. [Google Scholar]
- 6.Westergaard M. Adv Genet. 1958;9:217–281. doi: 10.1016/s0065-2660(08)60163-7. [DOI] [PubMed] [Google Scholar]
- 7.Farbos I, Oliveira M, Negrutiu I, Mouras A. Sex Plant Reprod. 1997;10:155–167. [Google Scholar]
- 8.Farbos I, Veuskens J, Vyskot B, Oliveira M, Hinnisdaels S, Aghmir A, Mouras A, Negrutiu I. Genetics. 1999;151:1187–1196. doi: 10.1093/genetics/151.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lardon A, Georgiev S, Aghmir A, Le Merrer G, Negrutiu I. Genetics. 1999;151:1173–1185. doi: 10.1093/genetics/151.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack T. Trends Plants Sci. 2001;6:310–316. doi: 10.1016/s1360-1385(01)01987-2. [DOI] [PubMed] [Google Scholar]
- 11.Hardenack S, Ye D, Saedler H, Grant S. Plant Cell. 1994;6:1775–1787. doi: 10.1105/tpc.6.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada S, Tasaka M. J Plant Res. 2002;115:411–417. doi: 10.1007/s10265-002-0061-7. [DOI] [PubMed] [Google Scholar]
- 13.Harrison J, Moller M, Langdale J, Cronk Q, Hudson A. Plant Cell. 2005;17:430–443. doi: 10.1105/tpc.104.028936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose TM, Schultz ER, Henikoff JG, Pietrokovski S, McCallum CM, Henikoff S. Nucleic Acids Res. 1998;26:1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zluvova J, Janousek B, Negrutiu I, Vyskot B. Genetics. 2005;170:1431–1434. doi: 10.1534/genetics.105.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long JA, Moan EI, Medford JI, Barton MK. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- 17.Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. Plant Cell. 1997;9:841–857. doi: 10.1105/tpc.9.6.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breuil-Broyer S, Morel P, de Almeida-Engler J, Coustham V, Negrutiu I, Trehin C. Plant J. 2004;38:182–192. doi: 10.1111/j.1365-313X.2004.02026.x. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher JC. BioEssays. 2002;24:27–37. doi: 10.1002/bies.10020. [DOI] [PubMed] [Google Scholar]
- 20.Laufs P, Peaucelle A, Morin H, Traas J. Development (Cambridge, UK) 2004;131:4311–4322. doi: 10.1242/dev.01320. [DOI] [PubMed] [Google Scholar]
- 21.Barton MK, Poethig RS. Development (Cambridge, UK) 1993;119:823–831. [Google Scholar]
- 22.Clark SE, Jacobsen SE, Levin JZ, Meyerowitz EM. Development (Cambridge, UK) 1996;122:1567–1575. doi: 10.1242/dev.122.5.1567. [DOI] [PubMed] [Google Scholar]
- 23.Endrizzi K, Moussian B, Haecker A, Levin JZ, Laux T. Plant J. 1996;10:967–979. doi: 10.1046/j.1365-313x.1996.10060967.x. [DOI] [PubMed] [Google Scholar]
- 24.Golz JF, Keck EJ, Hudson A. Curr Biol. 2002;12:515–522. doi: 10.1016/s0960-9822(02)00721-2. [DOI] [PubMed] [Google Scholar]
- 25.Groot EP, Sinha N, Gleissberg S. Plant Mol Biol. 2005;58:317–331. doi: 10.1007/s11103-005-4548-1. [DOI] [PubMed] [Google Scholar]
- 26.Souer E, Van Houwelingen A, Kloos D, Mol J, Koes R. Cell. 1996;85:159–170. doi: 10.1016/s0092-8674(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 27.Weir I, Lu J, Cook H, Causier B, Schwarz-Sommer Z, Davies B. Development (Cambridge, UK) 2004;131:915–922. doi: 10.1242/dev.00993. [DOI] [PubMed] [Google Scholar]
- 28.Takada S, Hibara K, Ishida T, Tasaka M. Development (Cambridge, UK) 2001;128:1127–1135. doi: 10.1242/dev.128.7.1127. [DOI] [PubMed] [Google Scholar]
- 29.Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MA, de Vries SC. Plant Cell. 2003;15:1563–1577. doi: 10.1105/tpc.012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aida M, Ishida T, Tasaka M. Development (Cambridge, UK) 1999;126:1563–1570. doi: 10.1242/dev.126.8.1563. [DOI] [PubMed] [Google Scholar]
- 31.Aida M, Vernoux T, Furutani M, Traas J, Tasaka M. Development (Cambridge, UK) 2002;129:3965–3974. doi: 10.1242/dev.129.17.3965. [DOI] [PubMed] [Google Scholar]
- 32.Stuurman J, Jaggi F, Kuhlemeier C. Genes Dev. 2002;16:2213–2218. doi: 10.1101/gad.230702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidner CA, Martienssen RA. Dev Biol. 2005;280:504–517. doi: 10.1016/j.ydbio.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 34.Matsunaga S, Uchida W, Kawano S. Plant Cell Physiol. 2004;45:795–802. doi: 10.1093/pcp/pch081. [DOI] [PubMed] [Google Scholar]
- 35.Zhang B, Pan X, Cannon CH, Cobb GP, Anderson TA. Plant J. 2006;46:243–259. doi: 10.1111/j.1365-313X.2006.02697.x. [DOI] [PubMed] [Google Scholar]
- 36.Janousek B, Siroky J, Vyskot B. Mol Gen Genet. 1996;250:483–490. doi: 10.1007/BF02174037. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JD, Higgins DG, Gibson TJ. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galtier N, Gouy M, Gautier C. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 39.Ferrandiz C, Sessions A. In: Arabidopsis: A Laboratory Manual. Weigel D, Glazebrook J, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2002. pp. 195–203. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.