Abstract
In the smut fungus Ustilago maydis, a tightly regulated cAMP signaling cascade is necessary for pathogenic development. Transcriptome analysis using whole genome microarrays set up to identify putative target genes of the protein kinase A catalytic subunit Adr1 revealed nine genes with putative functions in two high-affinity iron uptake systems. These genes locate to three gene clusters on different chromosomes and include the previously identified complementing siderophore auxotroph genes sid1 and sid2 involved in siderophore biosynthesis. Transcription of all nine genes plus three additional genes associated with the gene clusters was also coregulated by iron through the Urbs1 transcription factor. Two components of a high-affinity iron uptake system were characterized in more detail: fer2, encoding a high-affinity iron permease; and fer1, encoding an iron multicopper oxidase. Fer2 localized to the plasma membrane and complemented an ftr1 mutant of Saccharomyces cerevisiae lacking a high-affinity iron permease. During pathogenic development, fer2 expression was confined to the phase of hyphal proliferation inside the plant. fer2 as well as fer1 deletion mutants were strongly affected in virulence. These data highlight the importance of the high-affinity iron uptake system via an iron permease and a multicopper oxidase for biotrophic development in the U. maydis/maize (Zea mays) pathosystem.
INTRODUCTION
The fungal pathogen Ustilago maydis causes smut disease of maize (Zea mays). The most prominent symptoms are plant tumors, and it is within these tumors that fungal hyphae proliferate and differentiate to diploid spores. U. maydis alternates between a unicellular yeast form and a dikaryotic filamentous form, which is infectious (Kahmann et al., 2000). The morphological transition is regulated by the a and b mating-type loci. The a locus codes for pheromones (Mfa1 and Mfa2) and the respective pheromone receptors (Pra1 and Pra2), whereas the b locus encodes a pair of transcription factors, bE and bW. These two proteins dimerize when derived from different alleles and then constitute the central regulator for sexual and pathogenic development (reviewed in Kahmann et al., 2000). Therefore, only cells that differ in a and b can form an infectious dikaryon.
Transcription of the a and b genes is controlled by a conserved mitogen-activated protein kinase cascade and through the cAMP pathway (Krüger et al., 1998; Kaffarnik et al., 2003; Müller et al., 2003). Both signaling pathways are required for discrete steps during pathogenic development (Gold et al., 1997; Müller et al., 1999, 2003; Krüger et al., 2000; Mayorga and Gold, 2001). cAMP signaling involves activation of the Ustilago adenylyl cyclase Uac1 via the G protein α subunit Gpa3 (Krüger et al., 1998). Subsequently, cAMP activates the cAMP-dependent protein kinase A (PKA) Adr1 by releasing the regulatory subunit Ustilago bypass of cyclase 1 (Ubc1) from the complex (Dürrenberger et al., 1998). Strains carrying deletions in either uac1 or gpa3 grow filamentously, indicating that cAMP represses filamentous growth (Barrett et al., 1993; Regenfelder et al., 1997). On the other hand, ubc1 mutants that mimic a situation of high internal cAMP level have a multiple budding phenotype resulting from a cytokinesis defect (Gold et al., 1994). Mutants in which cAMP signaling is abolished are apathogenic, and the same holds for ubc1 mutants. This demonstrates that regulated levels of PKA activity are essential for disease progression (Barrett et al., 1993; Gold et al., 1997; Dürrenberger et al., 1998).
cAMP signaling plays a key role during pathogenic development and cell morphogenesis in a variety of fungi, including human and plant pathogens (Alspaugh et al., 1997; Borges-Walmsley and Walmsley, 2000; D'Souza et al., 2001; Lee et al., 2003). U. maydis is one of the few fungal pathogens in which several downstream target genes of the cAMP pathway have been identified. These include the high mobility group domain transcription factor pheromone response factor 1 (Prf1), which binds to pheromone response elements. Prf1 is phosphorylated by Adr1 and is then able to activate transcription of the pheromone and receptor genes (Kaffarnik et al., 2003). Moreover, Ustilago kinase B--related 1 (Ukb1), a putative Ser/Thr protein kinase containing 30 putative PKA phosphorylation sites, is proposed to be a PKA target. Ukb1 has a role in lateral budding and filamentous growth (Abramovitch et al., 2002). Similar to ubc1 mutants, ukb1 mutant strains infect their host but fail to induce tumors and are unable to complete sexual development (Abramovitch et al., 2002). Another direct target of PKA is the hyphal growth locus protein 1, which acts as a regulator for the switch between budding and filamentous growth (Dürrenberger et al., 2001). The identification of these genes is beginning to shed light on the processes that are regulated by cAMP in U. maydis.
Because of the general importance of cAMP signaling in pathogenesis, genome-wide expression profiling, serial analysis of gene expression, and PCR-select methods are currently applied to detect genes whose transcriptional profiles are affected by cAMP signaling (Labudova and Lubec, 1998; Irie et al., 2003, Lian et al., 2005). In U. maydis, serial analysis of gene expression recently was used to compare expression profiles of ubc1 and adr1 mutants, revealing a novel connection between cAMP and phosphate metabolism (Larraya et al., 2005). Here, we describe the use of genome-wide arrays for the detection of genes that are regulated via the cAMP pathway in U. maydis. We show that a large number of genes change expression upon induction of adr1. In this article, we concentrate on a set of coregulated genes that comprise three gene clusters involved in iron acquisition. One of these clusters is the previously identified ferrichrome biosynthesis gene cluster containing the complementing siderophore auxotroph genes sid1 and sid2 (Yuan et al., 2001). U. maydis is known to produce two siderophores, ferrichrome and ferrichrome A (Budde and Leong, 1989). Ferrichrome complexed iron is taken up and likely serves as an intracellular iron store, whereas ferrichrome A is thought to deliver iron to a membrane-bound iron reductase (Ecker and Emery, 1983; Ardon et al., 1997, 1998; Haas, 2003). In U. maydis, siderophore synthesis begins with the hydroxylation of Orn, and this step is catalyzed by an l-Orn N5-monooxygenase encoded by sid1 (Mei et al., 1993). sid2 is a siderophore synthetase responsible for the synthesis of ferrichrome (Yuan et al., 2001). Both genes are under negative regulation by the iron-responsive GATA transcription factor Urbs1 (for U. maydis regulator of biosynthesis of siderophores) (An et al., 1997). Because sid1 deletion mutants produce no siderophores and are not affected in virulence, iron acquisition during the biotrophic phase of U. maydis was postulated to use a different uptake system (Mei et al., 1993). Here, we show that the additional genes in the three identified gene clusters are also subject to regulation by iron through the Urbs1 transcription factor. Most importantly, we demonstrate that genes in one of the clusters code for a permease-based high-affinity iron uptake system that is required for virulence in U. maydis.
RESULTS
Identification of Coregulated Gene Clusters Involved in Iron Uptake
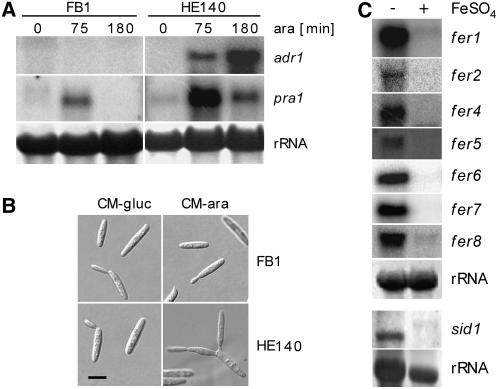
To identify genes whose expression depends on or is affected by the PKA Adr1, we performed a comparative genome-wide expression analysis. To this end, strain HE140 was generated, which expresses adr1 encoding the catalytic subunit of the PKA under the control of the arabinose-inducible crg1 promoter (Bottin et al., 1996). This experimental design was chosen because Δadr1 strains have a severe morphological phenotype and are unstable (Dürrenberger et al., 1998, 2001). We chose to overexpress Adr1 rather than adding cAMP to avoid the additional induction of catalytic subunits of other PKAs present in U. maydis (Dürrenberger et al., 1998). The inducible adr1 allele was integrated in a single copy into the ip locus of wild-type strain FB1 without affecting the native adr1 allele. To assess the function of the introduced allele, the expression of adr1 was compared with that of pra1 by RNA gel blot analysis (Figure 1A). Expression of pra1 is known to be activated by cAMP (Krüger et al., 1998; Kaffarnik et al., 2003) and served as a control. After induction of the crg1 promoter, adr1 expression increased significantly over time in strain HE140 but remained barely detectable in the FB1 control strain (Figure 1A). This finding illustrates that data were collected under conditions in which adr1 was overexpressed. pra1 was strongly upregulated 75 min after induction of adr1 (Figure 1A) and declined at 180 min after induction. In FB1 wild-type cells subjected to the same shift conditions, one could also observe an increase in pra1 transcript levels after 75 min in arabinose (Figure 1A). This increase was transient, however, and disappeared after 180 min (Figure 1A). This suggests that the transient peak seen in HE140 and in FB1 is attributable to the medium shift. One possibility is that this reflects the release from catabolite repression. Because the pheromone response factor prf1, the main regulator of pra gene expression, is transcribed with the same efficiency in medium containing glucose or arabinose (Hartmann et al., 1999), one would have to assume additional regulatory circuits in such a scenario. Also, cells lacking the regulatory subunit ubc1 show high levels of a gene expression in glucose-containing medium, which makes it unlikely that glucose is repressing those genes that are activated through the cAMP pathway. At 180 min after induction, 7% of all HE140 cells had started to form a second bud at the same pole or showed lateral buds (Figure 1B; data not shown), two phenotypic alterations induced by high cAMP levels (Gold et al., 1994). At later time points, nearly all cells displayed this phenotype (data not shown). In FB1, no changes in morphology were noted at any time point (Figure 1B). These results demonstrate that strain HE140 is suited to follow the events occurring after activation of the cAMP pathway.
Figure 1.
Characterization of Strain HE140 and Repression of Iron Uptake Cluster Genes by Iron.
(A) Induction of the crg1 promoter in U. maydis strain HE140 (a1b1[Pcrg1:adr1]ips) by arabinose (ara) induces adr1 and pra1 gene expression. Total RNA (10 μg) from the wild type (FB1) and strain HE140 isolated at 0, 75, and 180 min after the shift to arabinose-containing medium was loaded in each lane and probed successively with pra1 and adr1. In the top panel, the same filter was probed successively with the genes indicated. In the middle panel, a separate filter was probed with sid1. Methylene blue staining of the rRNA is shown in the bottom panel as a loading control for the two separate filters used.
(B) Arabinose induction of U. maydis strain HE140 leads to unipolar budding. Light microscopy is shown for wild-type FB1 and HE140 strains grown in complete medium (CM) (Holliday, 1974) supplemented with 1% glucose (left) and shifted for 180 min to CM containing 1% arabinose (right). Bar = 5 μm.
(C) Iron represses the expression of genes localized in the high-affinity iron uptake clusters. Total RNA (10 μg) from wild-type strain FB1 grown in minimal medium (Sundström, 1964) without (−) and with (+) the addition of 10 μM FeSO4 was used in each lane. Blots were successively probed with gene-specific probes as indicated at right. Methylene blue staining of the rRNA is shown as a loading control at bottom.
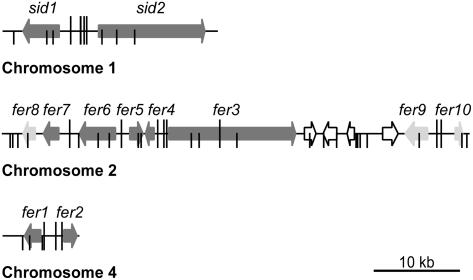
For expression profiling, Affymetrix arrays representing 93% of all U. maydis genes were used (see Methods). Except for the zero time points, at which only two biological replicates were used, all subsequent time points were analyzed in biological triplicates to exclude variations in culture conditions and handling. HE140 and FB1 strains were grown to log phase (OD600 = 0.5) in CM containing glucose. Expression of adr1 was activated by shifting the cells to CM containing arabinose. Before induction as well as 75 and 180 min after induction, cells were harvested and analyzed. Among a set of 847 genes that were differentially regulated in HE140 and FB1 (see Supplemental Figure 1 and Supplemental Table 1 online), we identified a subset of 407 coregulated genes (see Methods for details; see Supplemental Figure 1 and Supplemental Table 1 online). The coregulated genes had in common that their expression values in strain HE140 stayed at a high level 75 min after switching on adr1 and declined at 180 min. In the wild-type strain FB1, expression levels of these genes declined significantly already after 75 min and increased again after 180 min (see Supplemental Figure 1 and Supplemental Table 1 online). In this group, we identified nine genes that not only exhibited coregulation but also were found to be located in three gene clusters on chromosomes 1, 2, and 4 of the U. maydis genome (Figure 2, dark gray arrows). Among the 10 genes were sid1 and sid2, already known to be part of the ferrichrome biosynthesis gene cluster (Yuan et al., 2001). Surprisingly, most of the other eight genes in the clusters were also predicted to have a function in iron uptake. They were designated fer (Fe-regulated) genes. In the cluster on chromosome 2, another putative siderophore peptide synthetase gene, fer3, with 25.9% identity to sid2, is present (Figure 2). We presume fer3 to encode the peptide synthetase catalyzing ferrichrome A biosynthesis. In the gene cluster on chromosome 2, we also found an acylase gene, fer5, that displays similarity to an acyltransferase found in the putative ferrichrome A biosynthetic gene cluster of Omphalotus olearius (Welzel et al., 2005) (Table 1). Fer5 could potentially carry out acylation of N5-hydroxy-l-Orn residues in ferrichrome A. Located between the fer3 and fer5 genes is fer4, encoding a putative enoyl-CoA isomerase (Figure 2, Table 1). This enzyme might be responsible for the trans configuration of the acyl chain attached to the hydroxy-Orn. In addition, two genes, fer6 and fer7, encoding major facilitator proteins are part of this cluster (Figure 2, Table 1). Fer7 is related to a likely siderophore transporter, Str3, from Schizosaccharomyces pombe (39% identity) and to Sit1p, a siderophore transporter from Saccharomyces cerevisiae (25% identity), suggesting a function in siderophore uptake. Fer6 shows high similarity with multispecific ATP binding cassette transporters and could have a role in the uptake of alternative ferric iron complexes. The two coregulated genes found on chromosome 1, fer1 and fer2, encode a putative ferroxidase and a high-affinity ferric permease, respectively (Figure 2, Table 1). Genes in all three clusters could conceivably encode components of two distinct high-affinity iron uptake systems (Kosman, 2003). We refer to these three gene clusters as iron uptake clusters.
Figure 2.
Physical Map of Three Coregulated Iron Uptake Clusters.
The coregulated genes localize to clusters on three chromosomes of U. maydis. Coding regions are represented by arrows indicating the direction of transcription. Dark gray arrows, coregulated genes involved in iron uptake identified by arabinose induction of HE140; light gray arrows, additional genes identified as being coregulated by iron and Urbs1; open arrows, genes not belonging to the coregulated iron uptake clusters (um01435, um01436, um01437, um01438, and um01442 on chromosome 2 and um00107 on chromosome 4). Urbs1 binding sites (G/TGATAA) as defined by An et al. (1997) are depicted as vertical bars. Sites carrying the extended consensus motif ATCG/TGATAAA/G identified in this study are marked with long vertical bars.
Table 1.
Annotation of Genes in the Iron Uptake Clusters
| Gene | No.a | Protein Length (Amino Acids)a | Putative Function | Closest Known Homolog (Accession No.), Organism | Amino Acid Identity (%)a |
|---|---|---|---|---|---|
| fer1b | um00105 | 629 | Iron multicopper oxidase | Lac1 (Q6E0Y2), Auricularia polytricha | 51.7 |
| fer2b | um10023 | 486 | High-affinity iron permease | CaFtr1 (Q5KJQ5), Cryptococcus neoformans | 35.7 |
| fer3c | um01434 | 4830 | Siderophore peptide synthetase | SidC (Q7Z8P4), Aspergillus nidulans | 33.6 |
| fer4c | um01433 | 274 | Enoyl-CoA isomerase/hydratase | BH1135 (Q9KDS6), Bacillus halodurans | 47.7 |
| fer5c | um01432 | 427 | Acyltransferase | Ato1 (AAX49354), Omphalotus olearius | 48.9 |
| fer6c | um01431 | 1397 | Multidrug resistance protein | Yor1 (Q5KED3), Cryptococcus neoformans | 39.7 |
| fer7c | um11339d | 659 | Siderophore transporter | Str3 (Q92341), Schizosaccharomyces pombe | 39.2 |
| fer8c | um11338de | 398 | Unknown | CNI00360 (Q5KC39), Cryptococcus neoformans | 35.0 |
| fer9f | um01439 | 715 | Ferric reductase | Fre3 (NP_015026), Saccharomyces cerevisiae | 24.3 |
| fer10f | um11873 | 117 | Unknown | RpsL (Q9Z9L9), Bacillus halodurans | 22.3 |
| fer11f | um01441 | 304 | Unknown | Psyr_1077 (Q4ZXI9), Pseudomonas syringae | 44.3 |
| sid1g | um10188h | 649 | l-Orn N5-monooxygenase | SidA (Q5SE95), Aspergillus fumigatus | 32.2 |
| sid2i | um10189 | 4114 | Ferrichrome siderophore peptide synthetase | SidC (Q7Z8P4), Aspergillus nidulans | 26.6 |
Data from the MIPS database (http://mips.gsf.de/genre/proj/ustilago/).
Accession number BK004082.
Accession number BK004083.
fer7 and fer8 are two independent genes as verified by RT-PCR.
Both annotated introns verified by RT-PCR and sequencing.
Accession number BN000978.
Accession number M98520.
Both annotated introns verified by RT-PCR.
Accession number U62738.
Genes in the Iron Uptake Clusters Are Iron Regulated
The sid1 and sid2 genes are repressed under high-iron conditions by the GATA factor Urbs1 (Mei et al., 1993; An et al., 1997; Yuan et al., 2001). Therefore, we analyzed the intergenic regions of the fer genes for potential Urbs1 binding sites (G/TGATAA). Elements with a perfect match to this site could be identified in the intergenic regions of all iron cluster genes (Figure 2). On these grounds, it was likely that the fer genes are regulated by iron through Urbs1. To study possible iron effects on fer gene expression, RNA gel blot analysis was performed with wild-type cells (FB1) grown either in low-iron medium or in the same medium in the presence of 10 μM FeSO4 (Sundström, 1964). sid1 was included as a control to confirm the observation that a transcript of 2.3 kb dominates under low-iron conditions, whereas a larger transcript of lower abundance is found in medium containing 10 μM iron (Mei et al., 1993) (Figure 1C). When cells were grown in low-iron medium, a high expression level was observed for all fer genes tested. By contrast, fer gene transcripts in RNA prepared from cells grown in the same medium containing iron could barely be detected (Figure 1C). This finding illustrates that the fer genes are repressed by iron, as was shown previously for sid1 and sid2 (Mei et al., 1993; Yuan et al., 2001). We can also infer from these results that the CM used to grow cells for the array experiments (see Supplemental Figure 1 and Supplemental Table 1 online) represents a medium limited in iron, which was experimentally verified.
To analyze a potential role of Urbs1 in regulating the identified fer genes, we isolated an urbs1 deletion mutant (BW12) and performed comparative array analysis. Transcript profiles of wild-type strain FB1 were generated from cells grown in CM in the presence and absence of iron, whereas transcript patterns of BW12 were determined from cells grown in CM in the presence of iron. Genes repressed by iron and Urbs1 were identified as being coregulated. A set of 67 genes showed a fold change > 1.5 in the comparison of FB1 and BW12 grown in the presence of 10 μM FeSO4 as well as in the comparison of FB1 grown with and without iron supplement (see Supplemental Table 2 online). For all fer genes and sid1 and sid2, regulation by Urbs1 could be shown (see Supplemental Table 2 online). This analysis also revealed the existence of three additional urbs1 and iron-repressed genes, of which one is located downstream of fer7 (termed fer8) and two are located downstream of fer3 (termed fer9 and fer10) (Figure 2, light gray arrows on chromosome 2). These genes are separated from fer3 by a group of four genes of unknown function, which are not regulated by iron (Figure 2). fer9 is related to the iron reductase frp1 from S. pombe, whereas fer10 is related to genes of unknown function in other organisms (Table 1). Except for fer8, which codes for a hypothetical protein (Table 1) whose relation to iron acquisition is unclear, the additional fer genes show perfect matches to the Urbs1 binding site in their respective promoter regions (G/TGATAA; Mei et al., 1993). However, the four genes in the clusters that are not regulated by Urbs1 also show this sequence motif either in their promoters or in their coding regions (Figure 2; see Discussion). In addition to the 12 urbs1 and iron-regulated genes in the three iron gene clusters, we identified 55 nonclustered genes as being urbs1 and iron regulated (see Supplemental Table 2 online).
cAMP and Iron Regulate the Expression of the Iron Gene Clusters
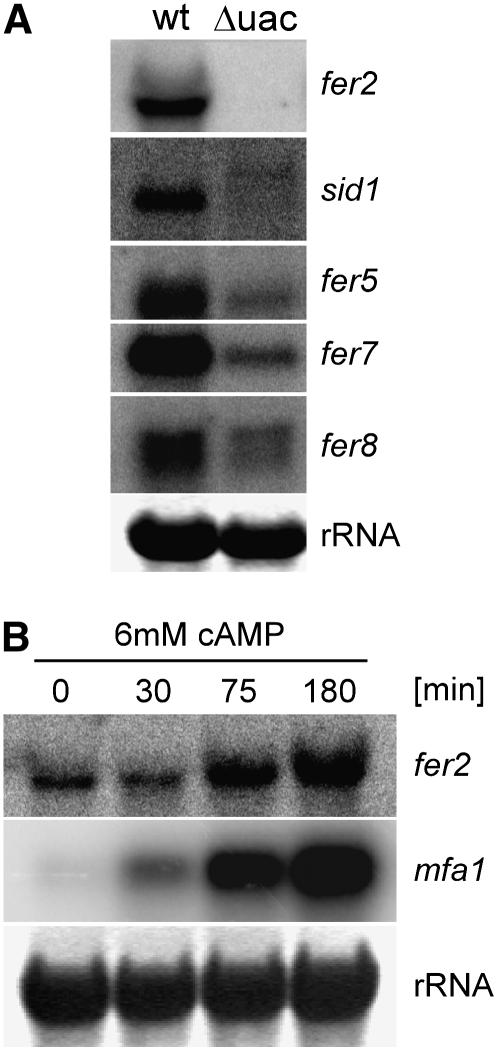
To learn more about the connection between repression by iron and activation by cAMP, we analyzed the expression of several fer genes and of sid1 in a Δuac1 mutant lacking adenylyl cyclase (Figure 3A). fer2 transcripts could not be detected in FB1Δuac1 strains, whereas expression of fer2 was seen in FB1 wild-type cells that were grown in the same medium (Figure 3A). These results show that fer2 expression completely depends on an intact cAMP pathway. For all other fer genes tested, as well as for sid1, lower transcript levels were seen in the Δuac1 strain compared with the wild type (Figure 3A), which illustrates that the full expression of these genes also requires an intact cAMP pathway. For sid1, low amounts of a 2.7-kb transcript were detected in FB1Δuac1, whereas a more abundant 2.3-kb transcript was visible in corresponding wild-type cells (Figure 3A). This finding reinforces the notion that CM is iron-limited and demonstrates that the smaller transcript seen under these iron-limiting conditions (Mei et al., 1993) requires the presence of an intact cAMP pathway. We also investigated the expression of the fer genes after cAMP feeding (Figure 3B; data not shown). In this experiment, the expression level of the pheromone gene mfa1, which is known to increase upon cAMP addition (Figure 3B), served as a control (Krüger et al., 1998). Upon addition of 6 mM cAMP, expression of fer2 was stimulated (Figure 3B). However, none of the other fer genes tested (fer1, -4, -5, -6, -7,and -8) responded with a significant induction (data not shown).
Figure 3.
Expression of Genes in the Iron Uptake Clusters Responds to cAMP Signaling.
(A) The expression of genes in the iron uptake clusters is reduced in a U. maydis strain lacking the regulatory subunit (Uac1) of the cAMP-activatable protein kinase C (Adr1). Total RNA (10 μg) isolated from wild-type strain FB1 (wt) and strain FB1Δuac1 (Δuac) grown in CM (Holliday, 1974) containing glucose was applied to each lane. The blot was probed sequentially with the probes indicated at right. Methylene blue staining of the rRNA is shown as a loading control at bottom.
(B) Expression of fer2 can be induced by cAMP feeding. RNA from wild-type strain FB1 grown in CM (Holliday, 1974) containing glucose was isolated at 0, 30, 75, and 180 min after stimulation with 6 mM cAMP. Total RNA (10 μg) was loaded in each lane. The blot was probed successively with probes against fer2 and mfa1. Methylene blue staining of the rRNA is shown as a loading control at bottom.
Characterization of the High-Affinity Iron Permease fer2
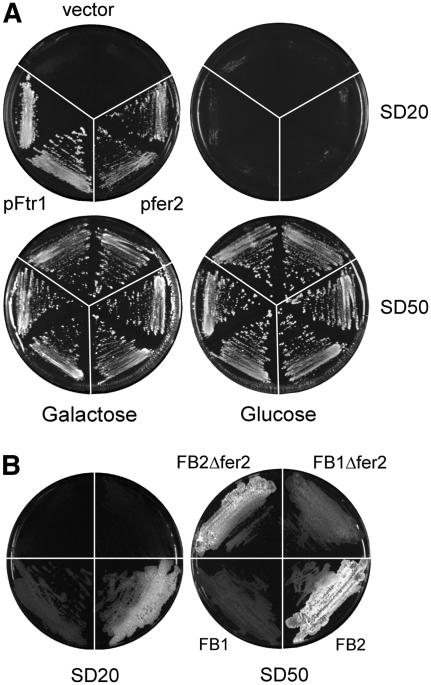
Because the high-affinity iron uptake system involving siderophores was shown to be dispensable for the pathogenicity of U. maydis (Mei et al., 1993), we considered the possibility that high-affinity uptake via an iron permease might be required for virulence. fer2 codes for a putative protein of 486 amino acids and shows significant homology with other fungal high-affinity iron permeases, such as Ftr2p from Candida albicans (44% identity) and Ftr1p from S. cerevisiae (35% identity). Inspection of the amino acid sequence identified a highly conserved REGLE motif in Fer2, which is located between amino acids 262 and 268 and is essential for iron uptake by iron permeases (Fang and Wang, 2002). To investigate whether Fer2 is functional, we tested the ability of U. maydis fer2 to complement an FTR1 mutant of S. cerevisiae. FTR1 mutant strains have an iron(III)-dependent growth defect that can be rescued by expressing FTR1 from a centromeric plasmid under the control of a galactose-inducible promoter (Ramanan and Wang, 2000). Transformants of strain BY4741ΔFTR1 carrying the empty vector YCplac111-G/T were unable to grow on low-iron plates even in the presence of galactose but grew well when iron was provided at higher concentrations (Figure 4A). The same strain transformed with either pfer2 or pFtr1 was unable to grow on low-iron plates containing glucose but showed growth on low-iron plates containing galactose (Figure 4A). This finding illustrates that U. maydis fer2 is able to complement the growth defect of an FTR1 strain of S. cerevisiae efficiently and thus encodes a functional iron permease.
Figure 4.
Complementation of the Iron-Dependent Growth Defect of the S. cerevisiae ΔFTR1 Mutant and the Iron-Dependent Growth Defect of U. maydis Δfer2 Strains.
(A) The iron-dependent growth defect of the S. cerevisiae ΔFTR1 mutant can be complemented by expression of fer2. The S. cerevisiae FTR1 mutant was transformed with empty vector YCplac111-G/T (vector), a plasmid expressing fer2 of U. maydis (pfer2), or a plasmid expressing FTR1 (pFtr1) under the control of the galactose-inducible GAL1 promoter. Two independent transformants were streaked on SD plates (Ramanan and Wang, 2000) containing FeCl3 (20 μM [SD20], top; or 50 μM [SD50], bottom) and galactose (2%; left) or glucose (2%; right). The plates were incubated for 3 d at 28°C.
(B) U. maydis Δfer2 strains are attenuated in growth on iron-limiting plates. Wild-type strains FB1 and FB2 and isogenic fer2 deletion strains were streaked on SD plates (Ramanan and Wang, 2000) containing glucose (2%) and FeCl3 to either 20 μM (SD20) or 50 μM (SD50). The plates were incubated for 3 d at 28°C. Differences in colony morphology between FB1 and FB2 strains and their derivatives are attributable to stronger filamentation of strains with the FB2 genetic background.
Pathogenic Development Is Attenuated in fer2 Deletion Mutants
To analyze the function of fer2, we constructed strains in which the entire open reading frame was replaced by a hygromycin resistance cassette by homologous recombination (FB1Δfer2#1 and FB2Δfer2#4). When compatible mixtures of Δfer2 strains were spotted on CM plates containing charcoal, dikaryotic filaments developed as efficiently as in compatible mixtures of wild-type strains (data not shown). However, when grown on low-iron plates, Δfer2 mutants were reduced in growth compared with the wild type (Figure 4B). This illustrates that the high-affinity iron uptake system based on the iron permease confers a selective growth advantage under iron(III)-limiting conditions.
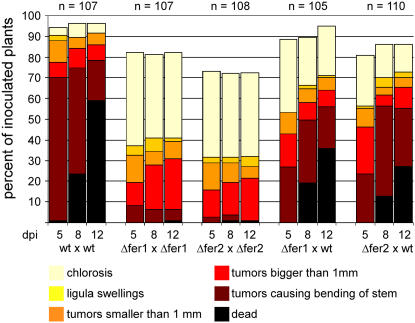
To investigate the consequences of the deletion of fer2 on pathogenic development, 5-d-old maize seedlings were inoculated with compatible mixtures of fer2 deletion strains. With respect to tumor formation, compatible mixtures of Δfer2 strains showed a significant reduction of disease symptoms compared with infections with compatible wild-type strains (Figure 5). After 12 d, on average 31.5 ± 1.8% of plants infected with a mixture of Δfer2 mutants showed tumor development, and only a single plant was killed. In comparison, 91.6 ± 5.1% of all plants infected with corresponding wild-type strains produced tumors, and in these infections plant death was observed in 59% of all cases. When a Δfer2 strain was crossed with a compatible wild-type strain, symptom development was restored almost to wild-type levels, illustrating complementation of the defect (Figure 5).
Figure 5.
U. maydis Strains Carrying Deletions in the fer2 and fer1 Genes Are Affected in Virulence.
Time course of disease progression in infections with wild-type and Δfer1 and Δfer2 deletion strains. Five-day-old maize seedlings were inoculated with a mixture of wild type (wt) strains FB1 and FB2, a mixture of compatible fer2 deletion mutants (Δfer2), a mixture of compatible fer1 deletion mutants (Δfer1), or mixtures of fer1 or fer2 deletion strains with the compatible wild-type strain. Disease progression was monitored at 5, 8, and 12 d after infection (dpi). Each plant was grouped into one of six categories according to the most severe symptom displayed. The categories were, in order of severity: chlorosis (yellow), ligula swellings (light orange), tumors smaller than 1 mm (dark orange), tumors larger than 1 mm (red), tumors causing bending of the stem (brown), and dead plants (black). The values shown are the sum of three independent experiments expressed as percentage of the total number of inoculated plants (n). In each experiment, 30 to 38 plants were inoculated for each strain combination.
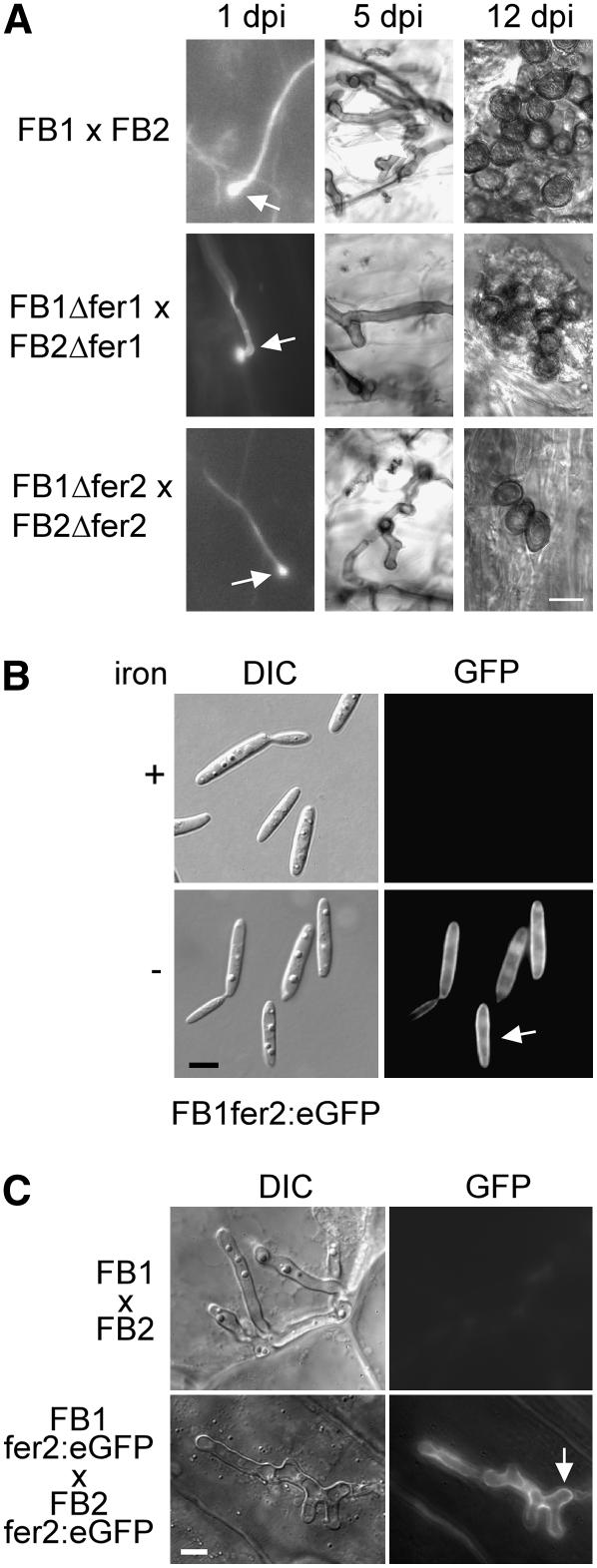
Fungal development after infection was also investigated microscopically. Although formation of appressoria-like structures was indistinguishable in infections with Δfer2 and wild-type strains (Figure 6A), 6 d after infection a reduction in fungal proliferation was observed in infections with the Δfer2 mutant compared with wild-type strains, and this difference in proliferation was maintained also at later stages (Figure 6A; data not shown). At day 12, wild-type strains had produced large amounts of mature spores, whereas in infections with fer2 mutant strains, spores were only rarely detected (Figure 6A) but were viable (data not shown). These results indicate that the Δfer2 mutants are attenuated during growth in planta. Δfer2 mutants were even more attenuated in virulence when maize yellow stripe mutants (ys1) lacking an iron siderophore transporter (Curie et al., 2001) were infected. Although wild-type strains could cause normal disease symptoms on these host plants (of 20 infected plants, all produced tumors at day 12 after infection), Δfer2 mutants were able to elicit anthocyanin production but completely failed with respect to tumor induction (of 21 infected plants, 17 showed anthocyanin induction but none developed tumors 12 or more days after infection).
Figure 6.
Biotrophic Development of Δfer2 Strains and Localization of the Fer2:eGFP Fusion Protein.
(A) Δfer1 and Δfer2 strains penetrate but show limited growth and rare spore formation. Plants were infected with a mixture of wild-type strains (FB1 × FB2), a mixture of fer1 deletion strains (FB1Δfer1 × FB2Δfer1), or a mixture of fer2 deletion strains (FB1Δfer2 × FB2Δfer2). Fungal structures were observed at 1, 5, and 12 d after infection (dpi) after staining with calcofluor and visualization with fluorescence microscopy (left panels), after staining with Chlorazole black E (middle panels), or without staining (right panels) and visualization by light microscopy (middle and right panels). At 1 d after infection, appressoria (arrows) were observed for all strain combinations (left panels). The sample showing an appressorium of the Δfer1 deletion strains was treated with chloroform before calcofluor staining. At 5 d after infection, fewer mycelial structures were observed in infections with fer1 and fer2 deletion strains than in infections using wild-type strains (middle panels). At 12 d after infection, fully melanized spores were readily observed in infections using wild-type strains but were rarely found in infections using fer1 or fer2 deletion strains (right panels). Bar = 10 μm for all panels.
(B) The Fer2:eGFP fusion protein is expressed under low-iron conditions and localizes to the plasma membrane. The U. maydis strain FB1fer2:eGFP was grown in minimal medium (Sundström, 1964) in the absence or presence of 10 μM FeSO4 (iron). Light microscopy images are shown in the left panel (differential interference contrast [DIC]), and fluorescence images are depicted at right (GFP). Bar = 5 μm.
(C) The Fer2:eGFP fusion protein is expressed at a specific stage during biotrophic growth. Plants were infected with a combination of wild-type strains (FB1 × FB2) or strains carrying the fer2:egfp fusion (FB1fer2:eGFP × FB2fer2:eGFP). Fungal hyphae within the plant tissue were visualized at 6 d after infection by light microscopy (differential interference contrast [DIC]; left panels) or by epifluorescence (GFP; right panels). Bar = 5 μm.
To reinforce the crucial role of the permease-based high-affinity iron uptake system for virulence in U. maydis. We also generated a deletion of the fer1 gene encoding a putative iron multicopper oxidase. Homologs of this gene product have been shown to reoxidize Fe2+ to Fe3+ in other systems and thereby provide the substrate for the permease (Kosman, 2003). When maize seedlings were coinoculated with a compatible mixture of fer1 deletion strains, virulence was reduced significantly (43.2 ± 2.3% compared with 91.6 ± 5.1% for the wild type; Figure 5). Virulence could be largely restored by coinoculation of a fer1 deletion strain with a compatible wild-type strain (Figure 5). fer1 mutants were attenuated in growth and spore formation (Figure 6A) and thus behave similar to Δfer2 mutant strains.
A Functional Fer2:GFP Fusion Protein Localizes to the Plasma Membrane and Is Expressed at a Specific Stage during Plant Colonization
To analyze the expression of fer2 during biotrophic growth and to study the localization of the fer2 gene product, we fused enhanced green fluorescent protein (eGFP) to the C terminus of Fer2. The respective strains FB1fer2:eGFP and FB2fer2:eGFP were as proficient in tumor induction as their progenitor strains, which illustrates that the fusion protein is functional (data not shown). In low-iron medium, the fusion protein localized mainly to the plasma membrane (Figure 6B). When cells were grown in medium supplemented with iron, no GFP fluorescence was detectable (Figure 6B). To study the expression pattern during infection, plants were inoculated with a mixture of FB1fer2:eGFP and FB2fer2:eGFP grown in YEPSL medium (see Methods), which provides iron in sufficient concentration to repress fer2 gene expression (data not shown). fer2 expression could not be detected in infection structures on the leaf surface and during biotrophic growth until day 5 after infection (data not shown). On day 6, characteristic staining of the plasma membrane was observed in lobed, branched hyphae (Figure 6C). This stage has been described to precede sporulation (Snetselaar and Mims, 1994; Banuett and Herskowitz, 1996). At later stages, expression of fer2 could no longer be detected (data not shown). These data indicate that starting with cells that are pregrown in iron-containing medium, fer2 gene expression is restricted to a narrow phase of fungal development within the plant tissue.
DISCUSSION
The use of genome-wide arrays coupled with the analysis of mutants has enabled us to identify a cluster of coregulated genes for two high-affinity iron uptake systems in U. maydis. Although the siderophore system is dispensable for pathogenesis (Mei et al., 1993), a ferroxidase/permease system was shown to be required for full virulence.
Iron Uptake Gene Clusters
The gene clusters identified in this study reside on three chromosomes and contain mostly genes with predicted functions in two high-affinity iron uptake systems. U. maydis is known to produce two siderophores, ferrichrome and ferrichrome A (Wang et al., 1989). Both are cyclic peptides that are nonribosomally produced and likely require specific transporters for excretion as well as plasma membrane permeases for uptake (Kosman, 2003). The genes in clusters on chromosomes 2 and 4 could potentially be involved in the biosynthesis and transport of siderophores. Of particular interest is fer3, encoding the putative peptide synthetase for the biosynthesis of ferrichrome A. To date, only three fungal peptide synthetase genes involved in siderophore biosynthesis have been functionally characterized (sid2 from U. maydis [Yuan et al., 2001], sidC from Aspergillus nidulans [Eisendle et al., 2003; Haas, 2003], and sib1 from S. pombe [Schwecke et al., 2006]). Based on the domain architecture of Fer3 (Schwecke et al., 2006), we anticipate that this enzyme is responsible for the synthesis of ferrichrome A. This is also supported by the finding of an iron-regulated putative ferrichrome A biosynthetic gene cluster in O. olearius (Welzel et al., 2005), which contains a peptide synthetase with higher identity to fer3 (29.8%) than to the ferrichrome synthetase sid2 (24.7%). Clustering of a siderophore peptide synthetase with an l-Orn N5-monooxygenase is a principle realized not only in U. maydis (Yuan et al., 2001) but also in S. pombe, Aureobasidium pullulans, and O. olearius (Haas, 2003; Welzel et al., 2005). In O. olearius, the cluster is extended by an acyltransferase gene predicted to be involved in the modification of N5-hydroxy-Orn, similar to the arrangement found in the cluster on chromosome 2 of U. maydis. Novel is the large gene cluster on chromosome 2 of U. maydis, which combines many genes for biosynthesis as well as for the transport of siderophores. Clustering of such activities has been observed only in A. pullulans, in which a single putative ATP binding cassette transporter gene maps between the siderophore synthetase and the l-Orn N5-monooxygenase genes (accession number U85909). Extensive clustering of siderophore biosynthesis and transport genes is a hallmark of pathogenic bacteria, in which the respective genes are found in pathogenicity islands (Schryvers and Stojiljkovic, 1999; Carniel, 2001; Rodriguez and Smith, 2003).
The third group of genes identified in this study is clustered on chromosome 1 and includes the genes for a high-affinity ferric permease as well as a putative iron multicopper oxidase. fer2 permease of U. maydis has no paralogs elsewhere in the genome and can complement the defects of a ΔFTR1 mutant of S. cerevisiae as efficiently as the yeast FTR1 gene, demonstrating that fer2 functions as a genuine ferric permease. Further experiments will be necessary to show whether Fe2+ or Fe3+ is the preferred substrate of fer2. The situation in which genes encoding an iron multicopper oxidase and a ferric permease are clustered is not unique to U. maydis; it is also found in Neurospora crassa, S. pombe, and Cryptococcus neoformans (Askwith and Kaplan, 1997; Lian et al., 2005). The iron permease and oxidase form a complex and are part of an indirect ferrous uptake system in which a relatively unspecific plasma membrane reductase accumulates and reduces Fe3+ present in the growth medium. The resulting Fe2+ is then reoxidized by the multicopper oxidase before transport by the permease (Kosman, 2003). Interestingly, in U. maydis, an iron-regulated putative reductase gene (fer9) is also contained in the cluster on chromosome 2.
Regulation of the Iron Cluster Genes
The iron cluster genes were identified because they appear to be coregulated when the PKA Adr1 is induced. The link to cAMP signaling could be substantiated for several fer genes tested as well as for sid1 (i.e., these genes displayed reduced transcript levels in a strain lacking adenylyl cyclase). However, fer2 was the only gene in which an increase in transcript levels was seen after cAMP feeding. In yeast, one of the three PKA catalytic subunits, Tpk2p, represses the transcription of genes involved in high-affinity iron uptake, which includes the genes encoding ferric reductases, an iron permease, as well as the putative siderophore iron transporter Sit1p (Robertson et al., 2000). For some of these genes, it has been shown that they are transcriptionally activated by AFT1 (for activator of ferrous transport) under conditions of iron deprivation (Yamaguchi-Iwai et al., 1996). In contrast with this positive regulation, sid1 and sid2 genes in U. maydis are negatively controlled by the GATA transcription factor Urbs1 in the presence of iron (Voisard et al., 1993; An et al., 1997). Urbs1-mediated repression is alleviated under low-iron conditions (Voisard et al., 1993). We show here that all genes in the iron gene clusters are also upregulated when iron is limiting and that this regulation occurs through Urbs1. Within the three iron clusters, 44 putative Urbs1 binding sites were identified, and a closer inspection revealed an extended conserved binding motif (ATCG/TGATAAA/G) for sites that are overrepresented in intergenic regions (Figure 2, long vertical bars). Therefore, we speculate that Urbs1 recognizes this more extended binding site. Most of the iron cluster genes contain this extended motif in their promoters (Figure 2). Only fer8 lacks this site, which may indicate that Urbs1 regulation can occur at a distance or may be indirect. Of the additional 55 Urbs1 and iron-regulated genes identified by array analysis, only four have the extended binding motif (see Supplemental Table 2 online). This finding could indicate that many of the genes, which are upregulated in urbs1 deletion strains under high-iron conditions, are not direct Urbs1 targets. It is conceivable that their expression in urbs1 mutants responds to uncontrolled iron uptake, which could induce a stress response. Such an observation has been made in SFU1 (for suppressor of ferric uptake) mutants of C. albicans, which lack the Urbs1 ortholog (Lan et al., 2004).
How could cAMP signaling and iron regulation be connected? Urbs1 transcription is not affected by iron (Voisard et al., 1993), which makes it likely that primary levels of control occur posttranscriptionally. Current models assume a direct role of iron in determining the activity of Urbs1 and related factors (Rutherford and Bird, 2004). In this respect, it may not be a coincidence that Urbs1 has eight putative PKA phosphorylation sites (PROSITE prediction; http://www.expasy.org). It is thus conceivable that the phosphorylation of Urbs1 could affect its DNA binding affinity or interaction with other regulatory proteins. In S. pombe, the Urbs1 ortholog Fe protein1 was shown to interact with Tup11 and Tup12 proteins, which are proposed to act as corepressors (Znaidi et al., 2004). The observed induction of fer2 after cAMP addition and the absence of fer2 transcripts in a Δuac1 strain would fit such a model. However, it is also clear that the other fer genes are subject to a more complex pattern of control, as they showed only reduced expression in the Δuac1 strain and none of them could be induced by cAMP feeding alone. During growth within the plant, fer2 expression could be detected only in a defined time window at ∼6 d after infection. Because the inoculum for this experiment was grown under iron-repressing conditions, we cannot exclude the possibility that cells may have used up their internal iron supplies before switching on the permease uptake system. In S. cerevisiae, this has been shown to require four cell doublings (Georgatsou and Alexandraki, 1994). We do not currently know whether the observed expression of fer2 at this stage is attributable to iron deprivation or reflects high internal cAMP levels.
Fer2 and Fer1 Are Virulence Factors
When maize plants are infected with a compatible combination of wild-type strains, tumor development is seen in >90% of infected plants and a high percentage of these plants die after a period of 12 d. In infections with Δfer2 as well as with Δfer1 mutants, fewer plants develop tumors, and these remain relatively small. As a consequence, plant death is rarely observed. On these grounds, both genes are virulence factors. fer2 and fer1 mutants are not affected in early development on the leaf surface; they form appressoria and penetrate. However, after 5 d, when wild-type dikaryons have heavily ramified the plant tissue, accumulations of fungal cells are rarely seen in the fer1 and fer2 mutants. As a likely result of this, both mutants produce significantly fewer spores than wild-type plants. Interestingly, Δfer2 strains are more virulent on a wild-type host than on the maize ys1 mutant, which has a defect in Fe(III) phytosiderophore uptake (Curie et al., 2001). This finding indicates that permease-based high-affinity iron uptake is sufficient for iron acquisition in wild-type strains even under conditions of low iron availability. Neither the high-affinity siderophore system nor low-affinity uptake involving a ferrous permease (for which um05420 is a possible candidate) can substitute for ferroxidation/permeation-mediated uptake in the fer2 mutant. Thus, the ferroxidation/permeation system is decisive for iron acquisition during plant colonization by U. maydis. This is demonstrated by the unaffected virulence of two different mutants unable to produce siderophores: the Δsid1 mutant, which lacks the first committed enzyme for siderophore biosynthesis and hence lacks both siderophores (Mei et al., 1993), and a mutant in which both siderophore synthetase genes are deleted (B. Winterberg, unpublished data). This finding could indicate that components of the system for iron uptake via siderophores (Winkelmann, 2002) are not expressed during biotrophic growth. In this scenario, the siderophore biosynthesis and transport genes would have to be under separate control from the genes encoding the reduction-based iron uptake system. One could also speculate that because Fe3+ exists mostly as phytochelate in the apoplast (Sparla et al., 1999), this might have to be converted to Fe2+ by the reductase and could then be channeled efficiently to the high-affinity oxidase/permease system.
At present, we also cannot formally rule out the possibility that fer2 could have additional roles besides providing iron for fungal growth in planta. It is conceivable that iron uptake is necessary to deplete iron from the environment to reduce the amount of iron available for the Fenton reaction, which could provide hydroxyl radicals from hydrogen peroxide, with potential harmful consequences to the fungus. However, diamino benzidine staining revealed no evidence for an accumulation of reactive oxygen species in tumor material from Δfer2 mutants (data not shown), despite the fact that such accumulations could readily be shown for mutant hyphae lacking a regulator for oxidative stress signaling (L. Molina and R. Kahmann, unpublished data).
So far, U. maydis is the only plant pathogenic fungus in which a ferroxidation/permeation iron uptake system is required for virulence. In this respect, U. maydis is similar to the human pathogen C. albicans, in which two high-affinity iron permeases, Ca FTR1 and Ca FTR2, were identified and one of these, Ca FTR2, was shown to be essential for pathogenicity in a mouse model for systemic infection (Ramanan and Wang, 2000). Interestingly, the siderophore uptake system of C. albicans has subsequently been shown to play a role in epithelial invasion and penetration (Heymann et al., 2002). In the human pathogen Aspergillus fumigatus, the biosynthesis of siderophores is also essential for virulence, whereas the reductive iron assimilation system is not needed (Schrettl et al., 2004). To date, Cochliobolus heterostrophus is the only phytopathogenic fungus in which the production of siderophores has been shown to be required for virulence (Lee et al., 2005; Oide et al., 2006). However, this unique position is likely to change when the mode of iron acquisition in other plant pathogens is analyzed. One of the intriguing questions remaining is why phytopathogenic fungi use different strategies for iron acquisition during plant colonization. We consider it possible that the fungal lifestyle adopted after infection (i.e., to grow as a biotroph or necrotroph) may ultimately determine in which form the iron present in the host plant can be acquired. Another interesting avenue for future research will be to elucidate at which stages of its life cycle U. maydis makes use of the siderophore-mediated iron uptake system.
METHODS
Strains and Culture Conditions
The Escherichia coli K-12 derivative DH5α (Bethesda Research Laboratories) and Top10 (Invitrogen) were used as hosts for cloning purposes. The Ustilago maydis strains used and constructed are listed in Table 2. U. maydis strains were grown at 28°C in YEPSL medium (1% yeast extract, 0.4% trypton, and 0.4% sucrose) in low-iron medium (Sundström, 1964) or in CM (Holliday, 1974). FeSO4 was added as indicated. For induction of the crg1 promoter, cells were grown at 28°C in CM containing 1% glucose to an OD600 of 0.5, washed once with CM containing 1% arabinose, and then resuspended in prewarmed CM containing 1% arabinose. To assess spore viability, tumor material was dried, homogenized in a mortar, treated with a solution of 0.75% copper sulfate for 15 min, washed, spread on PD plates (3.9% potato dextrose agar and 0.01 M Tris-HCl, pH 8.0), and incubated for 2 d at 28°C. To test for mating, strains were cospotted on CM plates containing 1% activated charcoal and incubated at room temperature for 24 h. Saccharomyces cerevisiae strain BY4741ΔFTR1 was propagated in YPD medium containing yeast extract (10 g/L), peptone (10 g/L), and glucose (20 g/L), and transformants harboring plasmids pFtr1, pfer2, or Ycplac111-G/T were grown on plates lacking Leu (Guthrie and Fink, 1991). SD medium was prepared according to Ramanan and Wang (2000) with the following modifications: ferrozine was added to a final concentration of 2 mM, and FeCl3 was added to final concentrations of 20 μM (SD20) or 50 μM (SD50) and contained either glucose (20 g/L) or galactose (20 g/L) as indicated.
Table 2.
Strains Used in This Study
| Strain | Genotype | Reference |
|---|---|---|
| Ustilago maydis | ||
| FB1 | a1b1 | Banuett and Herskowitz (1989) |
| FB2 | a2b2 | Banuett and Herskowitz (1989) |
| SG200 | a1mfa2bE1bW2 | Bölker et al. (1995) |
| FB1Δuac1 | a1b1Δuac1 | Krüger et al. (1998) |
| HE140 | a1b1[Pcrg1:adr1]ips | This work |
| FB1Δfer2 | a1b1Δfer2 | This work |
| FB2Δfer2 | a2b2Δfer2 | This work |
| FB1fer2:eGFP | a1b1fer2:eGFP | This work |
| FB2fer2:eGFP | a2b2fer2:eGFP | This work |
| FB1Δfer1 | a1b1Δfer1 | This work |
| FB2Δfer1 | a2b2Δfer1 | This work |
| BW12 | a1b1Δurbs1 | This work |
| Saccharomyces cerevisiae | ||
| BY4741ΔFTR1 | MATaHIS3Δ1LEU2Δ0MET15 Δ0URA3Δ0FTR1∷KanMX | Winzeler et al. (1999) |
Plant Infection Assays
Plant infections were done as described (Gillissen et al., 1992) with the maize (Zea mays) variety Early Golden Bantam. U. maydis strains were grown in YEPSL medium to an OD600 of 0.6 to 0.9, harvested, and resuspended in water to an OD600 of 2. For each mutant, between 30 and 40 plants (5-d-old seedlings) were syringe-infected with ∼200 μL of the cell suspension. Plants were kept in a greenhouse (16 h of light, 28 to 32°C; 8 h of dark, 20°C), and symptoms were scored at different times after infection. Categories for disease rating were as follows: no symptoms, chlorosis, ligula swellings, small tumors of <1 mm in diameter, tumors of >1 mm in diameter that do not affect the growth axis of the plant, tumors of >1 mm in diameter that cause bending of infected stems, and dead plants. Based on the strongest symptoms, each infected plant was placed in one of these categories. Because of the high inoculum and the use of young maize seedlings for the infection, a high percentage of plants are killed by wild-type strains. Each plant infection experiment was done three times independently. Percentages given in Figure 5 represent averages of three experiments.
DNA and RNA Procedures
Standard molecular techniques followed Sambrook et al. (1989). U. maydis chromosomal DNA was isolated according to Hoffman and Winston (1987). Transformation of U. maydis followed the protocol of Schulz et al. (1990). Transformation of S. cerevisiae was done according to Guthrie and Fink (1991). RNA was isolated following the TRIZOL reagent protocol (Invitrogen). Probes for detecting transcripts from the iron gene clusters were generated by PCR and verified by diagnostic digestions. To generate probes for the fer genes, a 1-kb fragment was generated using the primer combination FL67 (5′-CGGACAGAACATCGGGTG-3′) and FL68 (5′-CCGTCCCCAAATCTGGTC-3′) for fer1; a 550-bp fragment was amplified with FL13 (5′-CTCACGACTCGCTTACCG-3′) and FL14 (5′-GAGGGTTCAGCTTCTCGG-3′) for fer2; a 650-bp fragment with primers FL61 (5′-CCCTTACCTCGCGTTCTG-3′) and FL62 (5′-GCGGAAGGCATCATCTGC-3′) for fer4; a 650-bp fragment with primers FL63 (5′-GAGACACCATGCAAGCCG-3′) and FL64 (5′-CCCTGTGAGACCGATCTC-3′) for fer5; a 1-kb fragment with primers FL31 (5′-CTCGCGGAACACTATGCG-3′) and FL32 (5′-CTGGGTGATGCCGAACAC-3′) for fer6; a 1.1-kb fragment with primers FL33 (5′-GCGTGATGCTGACGCTTC-3′) and FL34 (5′-GATCGTGCGTTACCTCCC-3′) for fer7; and an 800-bp fragment with primers FL65 (5′-GGGCGTCAAATGGGCTCAG-3′) and FL66 (5′-CGCTCGATCGATTCCCAG-3′) for fer8. To detect sid1, we generated an 800-bp fragment using primers FL5 (5′-GACCTCCTAGGTATCGGC-3′) and FL6 (5′-GCGCGGAAGATCATGGTG-3′). As a probe for adr1, a 1.2-kb NcoI-XhoI fragment was isolated from pMF35 (Kaffarnik et al., 2003). Probes for pra1 and mfa1 were as described (Müller et al., 2003). For radioactive labeling of DNA, the Megaprime DNA labeling kit (New England Biolabs) was used. Detection and quantification of the signals were done with the help of a STORM PhosphorImager and the ImageQuant program (Molecular Dynamics).
Plasmids and Plasmid Construction
pHEcrg:adr1
pHEcrg:adr1 was constructed by a three-fragment ligation: a 7.7-kb NdeI-EcoRI fragment from pRU11 (Brachmann et al., 2001) containing the carboxin resistance cassette and the crg1 promoter fragment, a 1.1-kb NdeI-XhoI fragment from pMF35 containing the 5′ coding region of the adr1 gene, and a 420-bp XhoI-EcoRI fragment from pMF35 containing the 3′ coding region of adr1 including the 3′ untranslated region.
pΔfer2
To generate pΔfer2, two 1.0-kb fragments containing the 5′ region and the 3′ region of the fer2 gene, respectively, were amplified by PCR using the primer combinations FL1 (5′-TTGTGGATGCAGGTGCGG-3′)/ FL2 (5′-CACGGCCTGAGTGGCCCCGTTGCCGACATGTTTGC-3′) and FL3 (5′-GTGGGCCATCTAGGCCGGGTACTGGTTTGCCGTC-3′)/FL4 (5′-CGAAGCGTCAGGTACGTG-3′). The fragments were subsequently digested with SfiI and ligated to the 2.7-kb SfiI-digested hygromycin resistance cassette from plasmid pMF1-h (Brachmann et al., 2004) before cloning into pCR4-TOPO.
pfer2:eGFP
For the generation of pfer2:eGFP, two 1.0-kb fragments comprising the 3′ region and the 3′ untranslated region of fer2 were amplified by PCR using the primer combinations FL52 (5′-CGCAGAGATGAAGAGGGC-3′)/ FL53 (5′-ATAGGCCGCGTTGGCCGAGAGGGTACTTGGGCCAG-3′) and FL54 (5′-ATAGGCCTGAGTGGCCAGAGTGAATGCGCCAGCG-3′)/FL56 (5′-GGAGGTCACTCGACGAGTC-3′), respectively. The fragments were subsequently digested with SfiI and ligated to the 3.7-kb SfiI-digested eGFP–hygromycin resistance cassette from plasmid pMF5-h (Brachmann et al., 2004) before cloning into pCR4-TOPO.
fer1 Deletion Construct
To generate a fragment for the deletion of fer1, the 5′ and 3′ flanking regions of fer1 were amplified with primer combinations HE148 (5′-CCGGCTCGCAAGTCAATC-3′)/HE149 (5′-CACGGCCTGAGTGGCCTTGCGAAGGTCGCCTAGG-3′) and HE150 (5′-GTGGGCCATCTAGGCCTGCATCATCACCGGCCTG-3′)/HE151 (5′-CTGAAGATCGTGGCGAGC-3′), respectively, before ligation to the 2.7-kb SfiI hygromycin resistance cassette of plasmid pMF1-h. The deletion construct was amplified (Kämper, 2004) with primers HE148 and HE151.
pΔUrbs1
For the construction of pΔUrbs1, two 1.1-kb fragments comprising the 5′ and 3′ untranslated regions of urbs1, respectively, were generated by PCR using the oligonucleotide combinations oBW35 (5′-ATGTGCGTGTTGAGAAGACC-3′)/oBW36 (5′-TGACGGCCATCTAGGCCTTCTTGTCGTCCACGTATCC-3′) and oBW37 (5′-TAGCGGCCTGAGTGGCCAAGAAGGTGCCGCGATCTGC-3′)/oBW38 (5′-TTTGGCCCAGCTAAGAACC-3′) and digested with SfiI before ligation to the 2.4-kb SfiI fragment of pBS-hyg (Kämper, 2004) containing the hygromycin resistance cassette. The 4.6-kb ligation product was cloned into pCR2.1-TOPO (Invitrogen).
pFtr1
pFtr1 was generated by inserting a 1.2-kb BamHI-SalI fragment from plasmid pGAD-Ftr1 carrying the complete FTR1 open reading frame into the respective sites of YCplac111-G/T. YCplac111-G/T is derived from plasmid YCplac111 (Gietz and Sugino, 1988) by insertion of the GAL1 promoter and a transcriptional terminator (H.D. Ulrich, unpublished data). pGAD-Ftr1 is a derivative of the two-hybrid vector pGAD424 (Clontech) carrying the complete FTR1 open reading frame (T. Albert and H.D. Ulrich, unpublished data).
pfer2
pfer2 was generated by inserting a 1.5-kb XbaI-PstI fragment containing the coding region of fer2 from plasmid pfer2ORF into YCplac111-G/T cleaved with the respective enzymes. pfer2ORF was generated by cloning the fer2 cDNA generated from strain FB1 by RT-PCR using primers HE131 (5′-TTCTGCAGGGGCGAAAATGGGCTT-3′) and HE134 (5′-TATCTAGACCCGGGAATGTCGGCAACGGGCAAC-3′) into pCR4-TOPO. All fragments generated by PCR were sequenced to exclude mutations.
Generation of U. maydis Strains by Homologous Recombination
Strain HE140 was generated by integration of the pHEcrg1:adr1 linearized with SspI into the ip locus (Loubradou et al., 2001). FB1Δfer2 and FB2Δfer2 were generated from FB1 and FB2, respectively, by transformation with the fer2 deletion construct generated by PCR from pΔfer2 using primers FL1 and FL4. The strains FB1fer2:eGFP and FB2fer2:eGFP were generated by transformation of the respective wild-type strains with a 5.7-kb EcoRI fragment from plasmid pfer2:eGFP. FB1Δfer1 and FB2Δfer1 were generated from FB1 and FB2, respectively, by transformation with the fer1 deletion construct. Strain BW12 was generated from FB1 by transformation with the 4.6-kb PCR-amplified deletion construct derived from pΔurbs1 using the oligonucleotides oBW35 and oBW38. Single-copy integration of all constructs as well as homologous recombination was verified for all strains by diagnostic PCR and DNA gel blot analysis.
Sample Preparation and Microarray Analysis
Briefly, total RNA (for isolation, see above) was purified applying the RNeasy Mini kit (Qiagen). Purified RNA (10 μg) was reverse-transcribed using the Superscript Choice system (Invitrogen). The cDNA generated was purified using the GeneChip sample cleanup module (Qiagen) and transcribed in vitro with the ENZO BioArray High Yields RNA transcript 21 labeling kit (ENZO Diagnostics) using biotinylated ribonucleotides. Copy RNA was purified using the GeneChip sample cleanup module (Qiagen) and subsequently fragmented according to standard protocols (Affymetrix). The quality of RNA, cDNA, and copy RNA fragments was analyzed with an Agilent Bioanalyzer 2100 and RNA 6000 Nano LabChips or DNA 7500 LabChips, respectively (Agilent Technologies).
DNA array analysis was performed with custom-designed Affymetrix chips (MPIUstilagoA). Probe sets were designed based on a 17.4-Mb map-based sequencing assembly of the U. maydis genome of strain 521 (Bayer CropScience sequence; http://www.broad.mit.edu/annotation/genome/ustilago_maydis/). For each predicted gene, 33 perfect match and 33 corresponding mismatch probes were designed covering a region of 800 bp at the 3′ ends. The U. maydis DNA arrays address ∼6300 of the 6902 predicted U. maydis genes. Genes not represented on the chip were recognized only after completion of the shotgun sequencing of U. maydis strain 521 by the Broad Institute (www.broad.mit.edu/annotation/fungi/ustilago_maydis/) and manual annotation by the Munich Information Center for Protein Sequences (MIPS). Probe sets for the individual genes have been integrated into the MIPS Ustilago maydis database and can be queried for individual genes at http://mips.gsf.de/genre/proj/ustilago/.
Hybridization was performed with fragmented copy RNA according to the Affymetrix protocol for eukaryotic targets on the Affymetrix custom array MPIUstilagoA. Subsequently, a three-stain procedure was performed according to the protocol EukGE-WS2 on a GeneChip Fluidics Station 400, which included signal amplification as well as washing steps (Affymetrix). Arrays were scanned twice using a GeneArray Scanner (Agilent/Affymetrix). The resulting image data were analyzed using the GeneChip Expression Analysis software (GCOS) Microarray Suite 5.0 (Affymetrix), using standard settings: Smooth factor, 100; Epsilon, 0.5; alpha1, 0.0575; alpha2, 0.0971; tau, 0.015; Gamma1H, 0.000423; Gamma1L, 0.000423; Gamma2H, 0.000627; Gamma2L, 0.000627; Perturbation, 1.1; TGT, 300. Further data analysis was performed using the Biconductor R package (http://www.bioconductor.org/). Expression values were converted to log2 (value + 1). Limma (Smyth, 2004) was used for the expression analysis of differentially regulated genes.
Using lmFit (Linear Model for Series of Arrays), a linear model was fitted to the log2 (value + 1) expression data for each probe, and contrasts.fit (Compute Contrasts from Linear Model Fit) was used to obtain coefficients and standard errors for contrasts of the coefficients of the original model. An empirical Bayes method, ebayes (Empirical Bayes Statistics for Differential Expression), was used to rank genes in order of evidence for differential expression. A table of the top-ranked genes from the linear model fit was extracted by topTable (Table of Top Genes from Linear Model Fit). The P values for the coefficients/contrasts of interest were adjusted for multiple testing by the Benjamini and Hochberg (1995) method fdr.
Genes with a corrected P value of <0.001 that were at least twofold regulated at either 75 or 180 min after induction were filtered by comparing HE140 and the FB1 control at the respective time points (biological triplicates each).
For the iron repression data set, genes were filtered with the following criteria: at least 1.5-fold regulated by the comparison of BW12 (FB1Δurbs1) with FB1 in the presence of 10 μM FeSO4, and at least 1.5-fold regulated by the comparison of FB1 in the presence and absence of 10 μM FeSO4 (biological duplicates each), and with a corrected P value of <0.05 in either of the two comparisons.
For cluster analysis, the dChip 1.2 software package (Li and Hung Wong, 2001a, 2001b; http://biosun1.harvard.edu/complab/dchip/) was used on gene lists generated by means of Bioconductor, and the expression values were calculated by MAS5.0 (dChip 1.2: hierarchical clustering) default settings: distance matrix, 1 − r (r is the Pearson correlation coefficient between standardized expression values), centroid linkage, gene ordering by cluster tightness (P value for significant cluster calls between genes, 0.001).
Sequence Analysis
Predicted amino acid sequences were analyzed using the programs BLASTP (Altschul et al., 1997), SMART (Schultz et al., 1998), and PFAM (Bateman et al., 2002).
Quantification of Iron
Iron quantification according to the method of Fish (1988) revealed that CM has an iron concentration of <0.8 μM, and the medium used to repress iron-responsive genes contained at least 10 μM FeSO4.
Microscopy
Sections for microscopy were cut with a sharp razor blade from infected leaf tissue and were immersed in water immediately before microscopic observation. Calcofluor staining of fungal structures on the leaf surface and Chlorazole black E staining of infected plant leaf samples were performed as described (Brachmann et al., 2003). Samples were observed using a Zeiss Axiophot microscope with differential interference contrast optics or by epifluorescence microscopy with a specific filter set (BP470/20, FT 493, BP 505-530) for eGFP fluorescence. Pictures were taken using a CCD camera (C4742-95; Hamamatsu). Image processing and measurements were performed using Axiovision 3.1 (Zeiss) and Canvas 7.0 (Deneba).
Accession Numbers
Sequence data from this article can be accessed through the MIPS Ustilago maydis database (http://mips.gsf.de/genre/proj/ustilago/) and can be found in the GenBank/EMBL data libraries under accession numbers BK004082, BK004083, and BN000978. Microarray data files were submitted to the National Center for Biotechnology Information GEO database and can be accessed under accession numbers GSE6037, GSE6038, and GSE6039.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Hierarchical Cluster Analysis of Adr1-Regulated Genes.
Supplemental Table 1. List of Differentially Regulated Genes upon Arabinose-Induced Adr1 Expression in HE140.
Supplemental Table 2. List of Iron- and Urbs1-Repressed Genes as Deduced by Microarray Analysis.
Supplementary Material
Acknowledgments
We thank Bayer CropScience for access to the U. maydis genome sequence before its public release. We thank N. von Wirén for maize ys1 seeds. We acknowledge M. Vranes, M. Scherer, and J. Pons for expert advice on microarray technology and data analysis. We are grateful to H.D. Ulrich, T. Albert, and M. Feldbrügge for advice and for supplying plasmids and strains. We thank K. Münch and E. Meyer for expert technical assistance. This work was supported through a grant from the German Ministry for Science and Education.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Regine Kahmann (kahmann@staff.uni-marburg.de).
Online version contains Web-only data.
References
- Abramovitch, R.B., Yang, G., and Kronstad, J.W. (2002). The ukb1 gene encodes a putative protein kinase required for bud site selection and pathogenicity in Ustilago maydis. Fungal Genet. Biol. 37 98–108. [DOI] [PubMed] [Google Scholar]
- Alspaugh, J.A., Perfect, J.R., and Heitman, J. (1997). Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev. 11 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An, Z., Mei, B., Yuan, W.M., and Leong, S.A. (1997). The distal GATA sequences of the sid1 promoter of Ustilago maydis mediate iron repression of siderophore production and interact directly with Urbs1, a GATA family transcription factor. EMBO J. 16 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon, O., Nudelman, R., Caris, C., Liebmann, J., Shanzer, A., Chen, Y., and Hadar, Y. (1998). Iron uptake in Ustilago maydis: Tracking the iron path. J. Bacteriol. 180 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon, O., Weizman, H., Liebmann, J., Shanzer, A., Chen, Y., and Hadar, Y. (1997). Iron uptake in Ustilago maydis: Studies with fluorescent ferrichrome analogs. An. Microbiol. (Rio J.) 143 3625–3631. [DOI] [PubMed] [Google Scholar]
- Askwith, C., and Kaplan, J. (1997). An oxidase-permease-based iron transport system in Schizosaccharomyces pombe and its expression in Saccharomyces cerevisiae. J. Biol. Chem. 272 401–405. [DOI] [PubMed] [Google Scholar]
- Banuett, F., and Herskowitz, I. (1989). Different a alleles are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl. Acad. Sci. USA 86 5878–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett, F., and Herskowitz, I. (1996). Discrete developmental stages during teliospore formation in the corn smut fungus, Ustilago maydis. Development 122 2965–2976. [DOI] [PubMed] [Google Scholar]
- Barrett, K.J., Gold, S.E., and Kronstad, J.W. (1993). Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol. Plant Microbe Interact. 6 274–283. [DOI] [PubMed] [Google Scholar]
- Bateman, A., Birney, E., Cerruti, L., Durbin, R., Etwiller, L., Eddy, S.R., Griffiths-Jones, S., Howe, K.L., Marshall, M., and Sonnhammer, E.L. (2002). The Pfam protein families database. Nucleic Acids Res. 30 276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57 289–300. [Google Scholar]
- Bölker, M., Genin, S., Lehmler, C., and Kahmann, R. (1995). Genetic regulation of mating and dimorphism in Ustilago maydis. Can. J. Bot. 73 320–325. [Google Scholar]
- Borges-Walmsley, M.I., and Walmsley, A.R. (2000). cAMP signalling in the pathogenic fungi: Control of dimorphic switching and pathogenicity. Trends Microbiol. 8 133–141. [DOI] [PubMed] [Google Scholar]
- Bottin, A., Kämper, J., and Kahmann, R. (1996). Isolation of a carbon source-regulated gene from Ustilago maydis. Mol. Gen. Genet. 253 342–352. [DOI] [PubMed] [Google Scholar]
- Brachmann, A., König, J., Julius, C., and Feldbrügge, M. (2004). A reverse genetic approach for generating gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 272 216–226. [DOI] [PubMed] [Google Scholar]
- Brachmann, A., Schirawski, J., Müller, P., and Kahmann, R. (2003). An unusual MAP kinase is required for efficient penetration of the plant surface by Ustilago maydis. EMBO J. 22 2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachmann, A., Weinzierl, G., Kämper, J., and Kahmann, R. (2001). Identification of genes in the bW/bE regulatory cascade in Ustilago maydis. Mol. Microbiol. 42 1047–1063. [DOI] [PubMed] [Google Scholar]
- Budde, A.D., and Leong, S.A. (1989). Characterization of siderophores from Ustilago maydis. Mycopathologia 108 125–133. [DOI] [PubMed] [Google Scholar]
- Carniel, E. (2001). The Yersinia high-pathogenicity island: An iron-uptake island. Microbes Infect. 3 561–569. [DOI] [PubMed] [Google Scholar]
- Curie, C., Panavienne, Z., Loulergue, C., Dellaporta, S.L., Briat, J.-F., and Walker, E.L. (2001). Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409 346–349. [DOI] [PubMed] [Google Scholar]
- D'Souza, C.A., Alspaugh, J.A., Yue, C., Harashima, T., Cox, G.M., Perfect, J.R., and Heitman, J. (2001). Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol. Cell. Biol. 21 3179–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürrenberger, F., Laidlaw, R.D., and Kronstad, J.W. (2001). The hgl1 gene is required for dimorphism and teliospore formation in the fungal pathogen Ustilago maydis. Mol. Microbiol. 41 337–348. [DOI] [PubMed] [Google Scholar]
- Dürrenberger, F., Wong, K., and Kronstad, J.W. (1998). Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc. Natl. Acad. Sci. USA 95 5684–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, D., and Emery, T. (1983). Iron uptake from ferrichrome A and iron citrate in Ustilago sphaerogena. J. Bacteriol. 155 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendle, M., Oberegger, H., Zadra, I., and Haas, H. (2003). The siderophore system is essential for viability of Aspergillus nidulans: Functional analysis of two genes encoding L-ornithine N5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC). Mol. Microbiol. 49 359–375. [DOI] [PubMed] [Google Scholar]
- Fang, H.-M., and Wang, Y. (2002). Characterization of iron-binding motifs in Candida albicans high-affinity iron permease CaFtr1p by site-directed mutagenesis. Biochem. J. 368 641–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, W.W. (1988). Rapid colorimetric micromethod for the quantitation of complexed iron in biological samples. Methods Enzymol. 158 357–364. [DOI] [PubMed] [Google Scholar]
- Georgatsou, E., and Alexandraki, D. (1994). Two distinctly regulated genes are required for ferric reduction, the first step of iron uptake in Saccharomyces cerevisiae. Mol. Cell. Biol. 14 3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, R.D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74 527–534. [DOI] [PubMed] [Google Scholar]
- Gillissen, B., Bergemann, J., Sandmann, C., Schröer, B., Bölker, M., and Kahmann, R. (1992). A two-component regulatory system for self/non-self recognition in Ustilago maydis. Cell 68 647–657. [DOI] [PubMed] [Google Scholar]
- Gold, S., Duncan, G., Barrett, K., and Kronstad, J. (1994). cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 8 2805–2816. [DOI] [PubMed] [Google Scholar]
- Gold, S.E., Brogdon, S.M., Mayorga, M.E., and Kronstad, J.W. (1997). The Ustilago maydis regulatory subunit of a cAMP-dependent protein kinase is required for gall formation in maize. Plant Cell 9 1585–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G.R. (1991). Guide to Yeast Genetics and Molecular Biology. Methods Enzymol. 194 13–37. [PubMed] [Google Scholar]
- Haas, H. (2003). Molecular genetics of fungal siderophore biosynthesis and uptake: The role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 62 316–330. [DOI] [PubMed] [Google Scholar]
- Hartmann, H.A., Krüger, J., Lottspeich, F., and Kahmann, R. (1999). Environmental signals controlling sexual development of the corn smut fungus Ustilago maydis through the transcriptional regulator Prf1. Plant Cell 11 1239–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann, P., Gerads, M., Schaller, M., Dromer, F., Winkelmann, G., and Ernst, J.F. (2002). The siderophore iron transporter of Candida albicans (Sit1p/Arn1p) mediates uptake of ferrichrome-type siderophores and is required for epithelial invasion. Infect. Immun. 70 5246–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman, C.S., and Winston, F. (1987). A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of E. coli. Gene 57 267–272. [DOI] [PubMed] [Google Scholar]
- Holliday, R. (1974). Ustilago maydis. In Handbook of Genetics, Vol. 1, R.C. King, ed (New York: Plenum Press), pp. 575–595.
- Irie, T., Matsumura, H., Terauchi, R., and Saitoh, H. (2003). Serial analysis of gene expression (SAGE) of Magnaporthe grisea: Genes involved in appressorium formation. Mol. Genet. Genomics 270 181–189. [DOI] [PubMed] [Google Scholar]
- Kaffarnik, F., Müller, P., Leibundgut, M., Kahmann, R., and Feldbrügge, M. (2003). PKA and MAPK phosphorylation of Prf1 allows promoter discrimination in Ustilago maydis. EMBO J. 22 5817–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann, R., Steinberg, G., Basse, C., Feldbrügge, M., and Kämper, J. (2000). Ustilago maydis, the causative agent of corn smut disease. In Fungal Pathology, J.W. Kronstad, ed (Dordrecht, the Netherlands: Kluwer Academic Publishers), pp. 347–371.
- Kämper, J. (2004). A PCR-based system for highly efficient generation of gene replacement mutants in Ustilago maydis. Mol. Genet. Genomics 271 103–110. [DOI] [PubMed] [Google Scholar]
- Kosman, D.J. (2003). Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 47 1185–1197. [DOI] [PubMed] [Google Scholar]
- Krüger, J., Loubradou, G., Regenfelder, E., Hartmann, A., and Kahmann, R. (1998). Crosstalk between cAMP and pheromone signalling pathways in Ustilago maydis. Mol. Gen. Genet. 260 193–198. [DOI] [PubMed] [Google Scholar]
- Krüger, J., Loubradou, G., Wanner, G., Regenfelder, E., Feldbrugge, M., and Kahmann, R. (2000). Activation of the cAMP pathway in Ustilago maydis reduces fungal proliferation and teliospore formation in plant tumors. Mol. Plant Microbe Interact. 13 1034–1040. [DOI] [PubMed] [Google Scholar]
- Labudova, O., and Lubec, G. (1998). cAMP up-regulates the transposable element mys-1: A possible link between signaling and mobile DNA. Life Sci. 62 431–437. [DOI] [PubMed] [Google Scholar]
- Lan, C.Y., Rodarte, G., Murillo, L.A., Jones, T., Davis, R.W., Dungan, J., Newport, G., and Agabian, N. (2004). Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53 1451–1469. [DOI] [PubMed] [Google Scholar]
- Larraya, L.M., Boyce, K.J., So, A., Steen, B.R., Jones, S., Marra, M., and Kronstad, J.W. (2005). Serial analysis of gene expression reveals conserved links between protein kinase A, ribosome biogenesis, and phosphate metabolism in Ustilago maydis. Eukaryot. Cell 4 2029–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B.N., Kroken, S., Chou, D.Y., Robbertse, B., Yoder, O.C., and Turgeon, B.G. (2005). Functional analysis of all nonribosomal peptide synthetases in Cochliobolus heterostrophus reveals a factor, NPS6, involved in virulence and resistance to oxidative stress. Eukaryot. Cell 4 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, N., D'Souza, C.A. and Kronstad, J.W. (2003). Of smuts, blasts, mildews, and blights: cAMP signalling in phytopathogenic fungi. Annu. Rev. Phytopathol. 41 03.1–03.29. [DOI] [PubMed] [Google Scholar]
- Li, C., and Hung Wong, W. (2001. a). Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc. Natl. Acad. Sci. USA 98 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., and Hung Wong, W. (2001. b). Model-based analysis of oligonucleotide arrays: Model validation, design issues and standard error application. Genome Biol. 2, RESEARCH0032. [DOI] [PMC free article] [PubMed]
- Lian, T., Simmer, M.I., D'Souza, C.A., Steen, B.R., Zuyderduyn, S.D., Jones, S.J.M., Marra, M.A., and Kronstad, J.W. (2005). Iron-regulated transcription and capsule formation in the fungal pathogen Cryptococcus neoformans. Mol. Microbiol. 55 1452–1472. [DOI] [PubMed] [Google Scholar]
- Loubradou, G., Brachmann, A., Feldbrügge, M., and Kahmann, R. (2001). A homologue of the transcriptional repressor Ssn6p antagonizes cAMP signalling in Ustilago maydis. Mol. Microbiol. 40 719–730. [DOI] [PubMed] [Google Scholar]
- Mayorga, M.E., and Gold, S. (2001). The ubc2 gene of Ustilago maydis encodes a putative novel adaptor protein required for filamentous growth, pheromone response and virulence. Mol. Microbiol. 41 1365–1379. [DOI] [PubMed] [Google Scholar]
- Mei, B., Budde, A.D., and Leong, S.A. (1993). sid1, a gene initiating siderophore biosynthesis in Ustilago maydis: Molecular characterization, regulation by iron, and role in phytopathogenicity. Proc. Natl. Acad. Sci. USA 90 903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, P., Aichinger, C., Feldbrügge, M., and Kahmann, R. (1999). The MAP kinase kpp2 regulates mating and pathogenic development in Ustilago maydis. Mol. Microbiol. 34 1007–1017. [DOI] [PubMed] [Google Scholar]
- Müller, P., Weinzierl, G., Brachmann, A., Feldbrügge, M., and Kahmann, R. (2003). Both mating and pathogenic development of the smut fungus Ustilago maydis are regulated by one MAP kinase cascade. Eukaryot. Cell 2 1187–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oide, S., Moeder, W., Krasnoff, S., Gibson, D., Haas, H., Yoshioka, K., and Turgeon, B.G. (2006). NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic Ascomycetes. Plant Cell 18 2836–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan, N., and Wang, Y. (2000). A high-affinity iron permease essential for Candida albicans virulence. Science 288 1062–1064. [DOI] [PubMed] [Google Scholar]
- Regenfelder, E., Spellig, T., Hartmann, A., Lauenstein, S., Bölker, M., and Kahmann, R. (1997). G proteins in Ustilago maydis: Transmission of multiple signals? EMBO J. 16 1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, L.S., Causton, H.C., Young, R.A., and Fink, G.R. (2000). The yeast A kinases differentially regulate iron uptake and respiratory function. Proc. Natl. Acad. Sci. USA 97 5984–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, G.M., and Smith, I. (2003). Mechanisms of iron regulation in mycobacteria: Role in physiology and virulence. Mol. Microbiol. 47 1485–1494. [DOI] [PubMed] [Google Scholar]
- Rutherford, J.C., and Bird, A.J. (2004). Metal-responsive transcription factors that regulate iron, zinc, and copper homeostasis in eukaryotic cells. Eukaryot. Cell 3 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., Frisch, E.F., and Maniatis, T. (1989). Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press).
- Schrettl, M., Bignell, E., Kragl, C., Joechl, C., Rogers, T., Arst, H.N., Jr., Haynes, K., and Haas, H. (2004). Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J. Exp. Med. 200 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers, A.B., and Stojiljkovic, I. (1999). Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32 1117–1123. [DOI] [PubMed] [Google Scholar]
- Schultz, J., Milpetz, F., Bork, P., and Ponting, C.P. (1998). SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA 95 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, B., Banuett, F., Dahl, M., Schlesinger, R., Schäfer, W., Martin, T., Herskowitz, I., and Kahmann, R. (1990). The b alleles of U. maydis, whose combinations program pathogenic development, code for polypeptides containing a homeodomain-related motif. Cell 60 295–306. [DOI] [PubMed] [Google Scholar]
- Schwecke, T., Göttling, K., Durek, P., Dueñas, I., Käufer, N.F., Zock-Emmenthal, S., Staub, E., Neuhof, T., Dieckmann, R., and von Döhren, H. (2006). Nonribosomal peptide synthesis in Schizosaccharomyces pombe and the architectures of ferrichrome-type siderophore synthetases in fungi. ChemBioChem 7 612–622. [DOI] [PubMed] [Google Scholar]
- Smyth, G.K. (2004). Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article 3. [DOI] [PubMed]
- Snetselaar, K.M., and Mims, C.W. (1994). Light and electron microscopy of Ustilago maydis hyphae in maize. Mycol. Res. 98 347–355. [Google Scholar]
- Sparla, F., Preger, V., Pupillo, P., and Trost, P. (1999). Characterization of a novel NADH-specific, FAD-containing, soluble reductase with ferric citrate reductase activity from maize seedlings. Arch. Biochem. Biophys. 363 301–308. [DOI] [PubMed] [Google Scholar]
- Sundström, K.-R. (1964). Studies of the physiology, morphology and serology of exobasidium. Symb. Bot. Ups. XVIII, 3.
- Voisard, C., Wang, J., McEvoy, J.L., Xu, P., and Leong, S.A. (1993). urbs1, a gene regulating siderophore biosynthesis in Ustilago maydis, encodes a protein similar to the erythroid transcription factor GATA-1. Mol. Cell. Biol. 13 7091–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Budde, A.D., and Leong, S.A. (1989). Analysis of ferrichrome biosynthesis in the phytopathogenic fungus Ustilago maydis: Cloning of an ornithine-N5-oxygenase gene. J. Bacteriol. 171 2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welzel, K., Eisfeld, K., Antelo, L., Anke, T., and Anke, H. (2005). Characterization of the ferrichrome A biosynthetic gene cluster in the homobasidiomycete Omphalotus olearius. FEMS Microbiol. Lett. 249 157–163. [DOI] [PubMed] [Google Scholar]
- Winkelmann, G. (2002). Microbial siderophore-mediated transport. Biochem. Soc. Trans. 30 691–696. [DOI] [PubMed] [Google Scholar]
- Winzeler, E.A., et al. (1999). Functional characterization of the Saccharomyces cerevisiae genome by gene deletion and parallel analysis. Science 285 901–906. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai, Y., Stearman, R., Dancis, A., and Klausner, R.D. (1996). Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 15 3377–3384. [PMC free article] [PubMed] [Google Scholar]
- Yuan, W.M., Gentil, G.D., Budde, A.D., and Leong, S.A. (2001). Characterization of the Ustilago maydis sid2 gene, encoding a multidomain peptide synthetase in the ferrichrome biosynthetic gene cluster. J. Bacteriol. 183 4040–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Znaidi, S., Pelletier, B., Mukai, Y., and Labbe, S. (2004). The Schizosaccharomyces pombe corepressor Tup11 interacts with the iron-responsive transcription factor Fep1. J. Biol. Chem. 279 9462–9474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.