Abstract
Cardiac pacemaker current, if, is generated by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. Our previous studies demonstrated that altered tyrosine phosphorylation can modulate the properties of both if and HCN channels. To assess a hypothesis that the intracellular tyrosine kinase Src may play a role in modulation by tyrosine phosphorylation of if, we cotransfected HEK293 cells with HCN4 and Src proteins. When HCN4 was cotransfected with a constitutively activated Src protein (Src529), the resultant voltage-dependent HCN4 activation was positively shifted (HCN4: V1/2 = −93 mV; Src529: V1/2 = −80 mV). The activation kinetics were accelerated at some potentials but not over the entire voltage range tested (eg, at −95 mV, τ_act(HCN4) = 3243 ms; τ_act(Src529) = 1113 ms). When HCN4 was cotransfected with a dominant negative Src protein (Src296), the HCN4 activation was shifted more negative to a smaller degree (HCN4: V1/2 = −93 mV; Src296: V1/2 = −98 mV; statistically insignificant) and the activation kinetics were slowed at most test potentials (eg, at −95 mV, τ_act(Src296) = 7396 ms). Neither Src529 nor Src296 significantly altered HCN4 current density. Coimmunoprecipitation experiments revealed that Src forms a complex with HCN4 in HEK293 cells and in rat ventricular myocytes. Our data provide a novel mechanism of if regulation by Src tyrosine phosphorylation.
Keywords: HCN channel, tyrosine phosphorylation, constitutively active Src kinase, rat myocytes
Tyrosine phosphorylation of ion channels represents an important regulatory mechanism governing ion channel functions.1 Our previous work has demonstrated that if in the rabbit sinus-atrial (SA) node is reduced by inhibition of tyrosine kinase activity with genistein or herbimycin A2 and enhanced by its stimulation with epidermal growth factor (EGF),3 which has also been demonstrated to increase heart rate.4,5 Reduced tyrosine kinase activity also inhibits if in rat ventricular myocytes.6 The mechanisms by which reduced tyrosine kinase activity inhibits if are different in SA node and ventricular myocytes. In response to reduced tyrosine kinase activity by the tyrosine kinase inhibitor genistein, SA node if is decreased without altered voltage dependence of activation. This has been explained by an inhibitory action of genistein on maximal current induced by hyperpolarization-activated cyclic nucleotide-gated (HCN4), a major HCN channel isoform in the SA node.6,7 In the ventricle, maximal if is reduced in association with a negative shift of activation, which is equivalent to the actions of genistein on the HCN2 channel protein, a prevalent isoform in the ventricle.6,7
To explore a specific tyrosine kinase involved in the modulation of if/HCN channel properties, we tested the hypothesis that the major intracellular tyrosine kinase, Src, regulates if by binding to HCN channel proteins. This hypothesis is based on the strategy used in the initial cloning of HCN1 using the Src SH3 domain as bait in a yeast 2-hybrid screen.8
A recent study reported that Src interacts with both heterologously expressed and native HCN channels in brain.9 Comparing HCN-induced currents in the presence and absence of a dominant negative Src, the authors concluded that Src phosphorylates HCN channels and this phosphorylation speeds the kinetics of activation. Our results, presented below, compare the effects of a constitutively active Src to a dominant negative form. These results suggest that in addition to effects on the kinetics, Src-mediated tyrosine phosphorylation can shift the voltage dependence of HCN4 channel activation to more positive potentials, an action that can have functional consequences in cardiac pacemaker activity.10
MATERIALS AND METHODS
Cell Culture and Transfection
HEK293 cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin. When cells approached confluence, they were seeded into 35-mm diameter dishes for HCN4 plasmid transfection using Lipofectamine (Invitrogen, Carlsbad, CA). Plasmids of HCN4 and Src529 or Src296 (Upstate Biotechnology, Lake Placid, NY) were co-transfected with the phrGFP (Stratagene, La Jolla, CA) to guide selection of cells expressing HCN channels 24 to 96 h after transfection cells with green fluorescence were selected for patch clamp experiments.
Whole Cell Patch Clamp Recordings
The isolated HEK293 cells were placed in a Lucite bath in which the temperature was maintained at 251 ± 1°C by a temperature controller. IHCN4 currents were recorded using the whole-cell patch clamp technique with an Axopatch–1D amplifier. The pipettes had a resistance of 2 to 4 MΩ when filled with solution containing (mmol/L) NaCl 6, K-aspartate 130, MgCl2 2, CaCl2 5, EGTA 11, and HEPES 10 (pH adjusted to 7.2 by KOH). The external solution contained (mmol/L) NaCl 120, MgCl2 1, HEPES 5, KCl 30, CaCl2 1.8, pH 7.4 (by NaOH). The Ito blocker, 4-AP (2 mmol/L), was added to the external solution to inhibit the endogenous transient potassium current, which can overlap with and obscure if tail currents. The data were acquired by CLAMPEX software (pClamp 8, Axon) for later analysis by CLAMPFIT (pClamp 8, Axon).
Rat Ventricle Preparation
The rat left ventricle was prepared as previously described.6,7 Briefly, adult rats (300–350 g) were euthanized by intraperitoneal injection of pentobarbital (1.2 g/kg) in accordance with protocols of the Institutional Animal Care and Use Committee at New York College of Osteopathic Medicine. Small pieces of epicardial ventricle from 6 rats were dissected and frozen in liquid nitrogen and ground to a fine powder using a mortar and pestle. About 1 g of powder was homogenized in 10 mL of heart membrane buffer (4 mmol/L HEPES, pH 7.0; 320 mmol/L sucrose plus protease inhibitors) and centrifuged at 2000g for 10 min. The homogenization was repeated and the supernatants pooled. The supernatants were then centrifuged at 100,000g for 1 h. The resulting pellet was resuspended in heart membrane buffer (0.5 mL/g heart) and analyzed by Western blot.
Immunochemistry
Immunoprecipitation
Cells were collected 24 to 48 h after transfection and lysed using RIPA buffer (10 mmol/L Tris, pH 7.4, 100 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L NaF, 20 mmol/L Na4P2O7, 2 mmol/L Na3VO4, 1% Triton X-100, 10% glycerol, 0.05% sodium dodecyl sulfate (SDS), 0.5% deoxycholate, 1 mmol/L PMSF, and protease inhibitor cocktail [Sigma, St Louis, MO]) and then gently sonicated. Samples were then subjected to coimmunoprecipitation by incubation with anti-HCN4 polyclonal antibody (Alpha Diagnostic International, San Antonio, TX), anti-Src polyclonal antibody (Upstate Biotechnology), and protein A/G beads. After incubation, the beads were boiled with SDS sample buffer and resolved by SDS-polyacrylamide gel electrophoresis (PAGE).
Western Blot Analysis
Western blots were performed by first resolving the samples by SDS–PAGE using 4% to 18% gradient gels (Bio-Rad). The samples were then transferred to nitrocellulose membranes (Amersham, Piscataway, NJ) and incubated with anti-HCN4 polyclonal antibody or anti-Src monoclonal antibody (GD11, Upstate Biotechnology). After incubation of the horseradish peroxidase (HRP)–conjugated secondary antibody, the blots were then incubated with ECL solution (Pierce Biotechnology, Rockford, IL) and exposed by autoradiography. Phosphotyrosine specific antibody, 4G10, was purchased from Upstate Biotechnology. For quantification of bands, the x-ray films were scanned using a densitometer (Molecular Devices, Sunnyvale, CA) and signals were measured using ImageQuaNT software. All protein experiments were repeated 3 times.
Data Analysis
Data are shown as mean ± SE. Western band intensities (Fig. 7B) are expressed as percentage of arbitrary unit corresponding to densitometric band intensity following background subtraction, and normalized to β-actin signal intensity from the same sample. Statistical comparisons were performed with 1-way ANOVA with P<0.05 considered to be statistical significance. A mixed 2-way ANOVA was applied to Figure 3 for statistical analysis.
FIGURE 7.
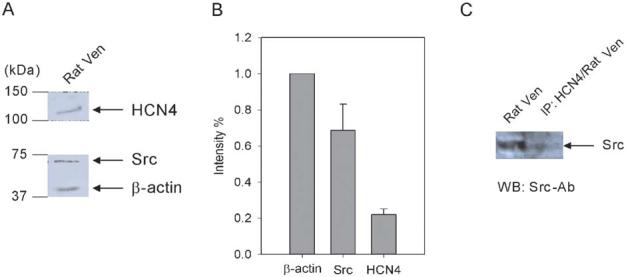
Interaction of HCN4 with Src in rat ventricle. A, Western blots of HCN4 and Src proteins in rat ventricle. The membranes were cut in half. The two halves were incubated with anti-HCN4 and anti-Src antibodies, respectively. The half membrane fraction incubated with anti-Src antibody was washed and reprobed with anti-β-actin antibody to reveal the β-actin signal. B, Src and HCN4 expression levels are normalized to β-actin, which is used as an internal control (n = 3). C, Right lane: rat ventricular sample was immunoprecipitated by a specific HCN4 antibody followed by Western blotting using a specific Src antibody; left lane: Western blotting of rat ventricle sample using a specific Src antibody.
FIGURE 3.
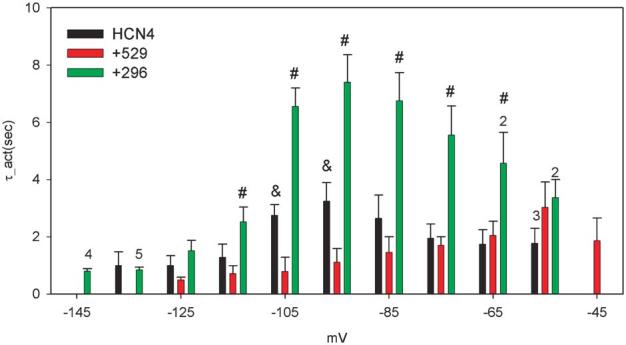
Activation kinetics of HCN4, HCN4+Src529, and HCN4+Src296. Activation kinetics were obtained by fitting the onset current traces to 30 or 40 s. Averaged kinetics data are presented from 8 cells expressing HCN4 and HCN4+Src529 and 10 cells expressing HCN4+296. Numbers above the bars indicate the number of cells analyzed at those potentials. #P <0.05 for HCN4 and HCN4+Src296 comparison; &P <0.05 for HCN4 and HCN4+Src529 comparison.
RESULTS
Electrophysiological Properties of HCN4 Channels Expressed in HEK293 Cells
To investigate the tyrosine phosphorylation by Src of HCN4 channels, we used a constitutively activated form of Src protein, Src529, and a dominant negative form of Src protein, Src296. Src529 is an Y529F mutant that prevents the normal downregulation of kinase activity and thus results in unregulated kinase activity of Src. Src296 is a double mutant (K296R/Y528F), which can interact with putative activators of Src, thus inhibiting endogenous Src kinase activity.
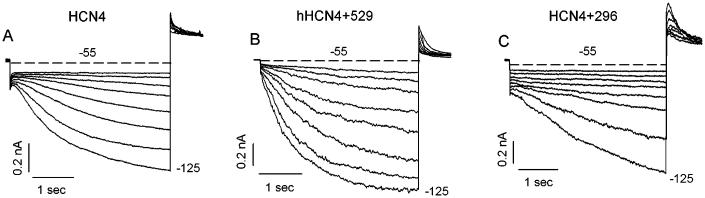
We first examined the activation of HCN4 channels expressed in HEK293 cells. Figure 1 shows that the currents, generated by HCN4 channels (Fig. 1A) and HCN4+Src529 (Fig. 1B) and HCN4+Src296 (Fig. 1C), were elicited by test pulses ranging from −55 to −125 mV for 4 s and then back to 20 mV for 2s. The holding potential was −10 mV. More negative potentials to −135 and −145 mV for 10 s were applied for cells expressing HCN4 and HCN4+296 because their activation saturated at potentials more negative than −125 mV. HCN4 activates slowly and the cells would not tolerate pulses sufficiently long to reach steady state. We therefore used the following approach to obtain an accurate estimate of steady-state activation. The onset current traces were fitted with a single exponential function to 30 s to allow estimates of steady-state current levels (Fig. 2A, upper panel); for currents (elicited by 4-s pulse) that activate extremely slowly, 10-s test pulses were applied and the corresponding traces were fit to 40 s to reach steady state (Fig. 2A, lower panel). The fitted current amplitudes were then divided by the driving force (the difference between test pulses and the reversal potential that was measured in each cell) to obtain the conductance at each test pulse. The activation curves were constructed by normalizing the conductance to its maximal value in response to the most negative test pulse. Averaging of the Boltzmann fits for each cell revealed the mean half-activations and the slope factors of −93 ± 5 and 15 ± 3 mV for HCN4 (n = 8), −80 ± 5 and 15 ± 2 mV for HCN4+Src529 (n = 8), and −98 ± 3 and 18 ± 2 mV for HCN4+Src296 (n = 10), respectively (Fig. 2B). The activation of HCN4 is positively shifted by Src529, which is statistically significant (P<0.05). However, the small negative shift of HCN4 activation induced by Src296 is not statistically significant (P >0.05). The difference in the half-activation of HCN4 induced by Src529 compared to Src296 is significant (P < 0.05) and represents the total effect of Src phosphorylation on activation of voltage dependence of 18 mV. Neither Src529 nor Src296 significantly alters the slope factor of HCN4 channel activation.
FIGURE 1.
An example of expression of HCN4 in HEK293 cells. From the holding potential of −10 mV, the cells were typically hyperpolarized to potentials ranging from −55 to −125 mV in 10 mV increment. Currents shown are for HCN4 alone (A), HCN4+Src529 (B), and HCN4+Src296 (C). The dashed lines represent the 0 currents.
FIGURE 2.
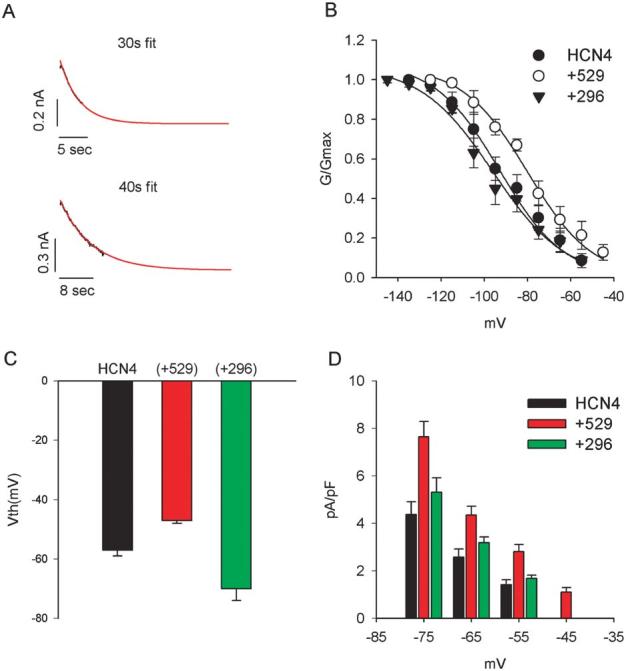
HCN4 activation. A, Method of fitting to obtain steady-state estimates of current activation (see text for details). B, Activation curves for HCN4, HCN4+Src529, and HCN4+Src296. The mean midpoints and slope factors of activation for HCN4 (−93 mV, −16 mV) and HCN4+Src529 (−80 mV, −16 mV) and HCN4+Src296 (−98 mV, −18 mV) were used to generate the fitting curves. C, Mean voltage thresholds for activation of HCN4, HCN4+Src529, and HCN4+Src296. D, Current density expressed as pA/pF at potentials in the range of −45 to −75 mV. There is more current with Src529 than HCN4 or HCN4+Src296 in this voltage range.
Although statistically, Src296 was not observed to significantly shift the half-activation of HCN4 to more negative potentials, there was a Src296-induced negative shift of voltage threshold (Vth) of activation (defined as the first potential at which the inward time-dependent current can be measured at amplitude larger than 20 pA). The mean values of Vth for HCN4, HCN4+529, and HCN4+Src296 are −57 ± 2 mV (n = 11),−47 ± 1 mV (n = 12), and −70 ± 4 (n = 14), respectively (Fig. 2C). Both shifts of Vth induced by Src529 and Src296 are statistically significant (P < 0.05). The Src529-induced positive shift of voltage threshold of HCN4 is in agreement with its similar effect on HCN4 activation curve (Fig. 2B). Because of the positive shift of Vth for HCN4 activation, larger steady-state current densities for Src529 are observed at potentials from −45 to −75 mV (Fig. 2D; P < 0.05 at each potential, n = 8 for HCN4 and HCN4+Src529, n = 10 for HCN4+Src296). No statistically significant increase in current density was observed at potentials negative to −75 mV.
We next analyzed the kinetics of HCN4 channels. Figure 3 shows the activation kinetics of HCN4, HCN4+Src529, and HCN4+Src296. Src529 accelerates HCN4 activation at −95 and −105 mV (P < 0.05, n = 8) but not at other membrane potentials. Src296 slows the HCN4 activation at potentials from −75 to −105 mV (P < 0.05; n = 8 for HCN4, n = 10 for HCN4+Src296). No significant difference in HCN4 activation kinetics was observed at potentials negative to −105 mV (P > 0.05 for Src529 and Src296 in comparison to HCN4; Fig. 2A). At −135 mV, the activation kinetics of HCN4 we observed are close to a recently reported value.9
Effects of Src529 and 296 on Initial Delay of Current Activation
In cardiac myocytes if undergoes a delay before activation.3,12,13 Figure 4 illustrates the effects of Src529 (Fig. 4B) and Src296 (Fig. 4C) on the delay in activation of HCN4 (Fig. 4A) currents recorded at −95 mV. To quantitate the difference in delay, we fit the current onset by a single exponential and extrapolated this fit to the level of current immediately observed after the voltage clamp step. One example of such a fit used to estimate the delay is provided in Figure 4D. This fitting procedure yielded delays of 0.185 ± 0.033(s) (n = 8), 0.044 ± 0.021(s) (n = 8), and 0.391 ± 0.078(s) (n = 10) for HCN4, HCN4+Src529, and HCN4+Src296, respectively (Fig. 4E). Both Src529-induced reduction and Src296-induced prolongation of the delay in HCN4 current activation are significant (P < 0.05).
FIGURE 4.
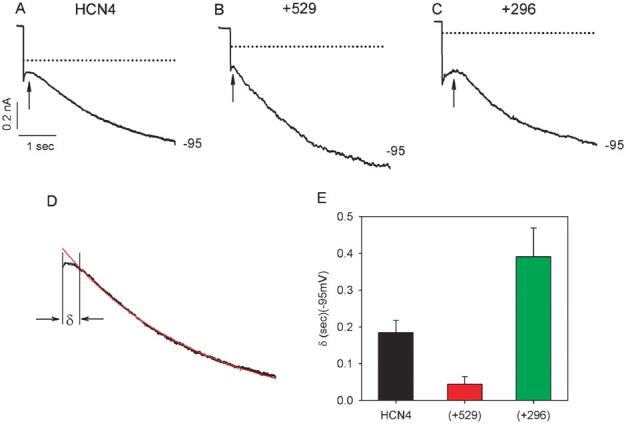
Initial delays (arrows) in HCN4 current activation at −95 mV. A typical set of current traces at −95 mV are shown for HCN4 (A), HCN4+Src529 (B), and HCN4+Src296 (C). Dotted lines are zero currents. D, Measurement of the initial delay: Delay is defined as the time difference between the beginning of current response to the voltage pulse and the beginning of the current trace that can be best fit by a single exponential function; labeled with the symbol δ. The capacitance current was excluded from the measurement. E, Mean delays are 0.185 ± 0.033(s) for HCN4 (n = 8), 0.044 ± 0.021(s) for HCN4+Src529 (n = 8), and 0.391 ± 0.078(s) for HCN4+Src296 (n = 10).
Interaction of Src with HCN4 Channels in HEK293 Cells
Src could modulate HCN4 properties in a direct way by physically binding to the channel protein or in an indirect manner mediated by other molecules. To distinguish between these 2 alternatives, we performed coimmunoprecipitation experiments in HEK293 cells transfected with HCN4 channels associated with or without Src529 or Src296.
Figure 5 provides a typical set of results. HCN4 channel proteins expressed in HEK cells were detected by a specific HCN4 antibody (Fig. 5A). No band was detected in untransfected cells. The specificity of the HCN4 antibody was tested against cells transfected with HCN2 (another isoform of the HCN channel family). No signal was observed. Figure 5B shows the expression of Src proteins. Weak but clear Src bands were detected in untransfected and HCN4-transfected HEK293 cells, indicating the presence of endogenous Src in HEK293 cells, which was previously reported.14 The levels of Src expression are enhanced in cells transfected with HCN4 plus Src529 and by cotransfection of HCN4 plus Src296. When cell lysate was immunoprecipitated (IP) with a Src antibody before detection by Western blot using the same antibody, then the Src protein band is present only in cells transfected with Src296 or HCN4 plus Src296 but not in untransfected or HCN4 transfected cells (Fig. 5C), indicating the required specificity of the Src antibody. When an HCN4 antibody is used to detect the signals from cell lysate immunoprecipitated with a Src antibody, HCN4 bands can be visualized in cells transfected with HCN4+Src529, but not in untransfected cells or in cells transfected with HCN4, HCN4+Src296, Src296, or Src529 (Fig. 5D), suggesting that HCN4 channel protein was in the HCN4/Src529 complex pulled down by the Src antibody. To confirm this finding, we immunoprecipitated the cell lysate with a HCN4 antibody followed by detection using a Src antibody. As shown in Figure 5E, Src signals are present in cells transfected with HCN4, HCN4+Src529, and HCN4+Src296. These data support the notion that Src can physically interact with HCN4 channel proteins.
FIGURE 5.
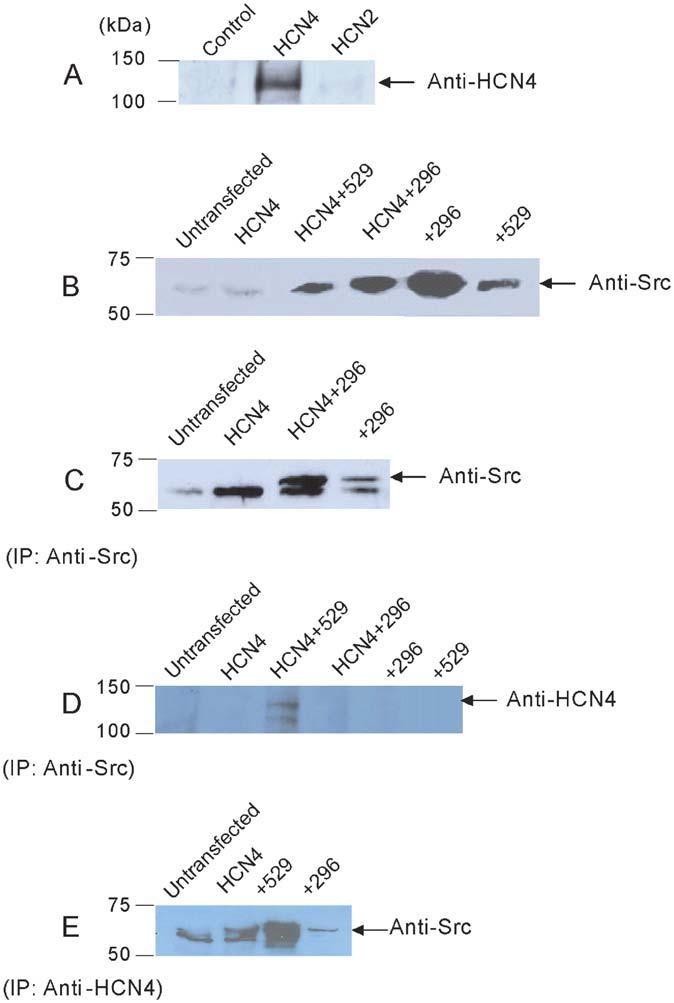
Immunoprecipitation of HCN4 with Src proteins in HEK cells. A, Western blot of HCN4 in untransfected, HCN4, and HCN2 cells. B, Western blot of Src in untransfected, HCN4, HCN4+Src529, HCN4+Src296, Src296, and Src529 cells. C, IP with anti-Src followed by Western blotting using anti-Src in untransfected, HCN4, HCN4+Src296, and Src296 alone cells. D, IP with anti-Src followed by Western blotting using anti-HCN4 in untransfected, HCN4, HCN4+Src529, HCN4+Src296, Src296, and Src529 cells. E, IP with anti-HCN4 followed by Western blotting using anti-Src in untransfected, HCN4, HCN4+Src529, and HCN4+Src296.
Tyrosine Phosphorylation of HCN4 Channel Proteins in HEK Cells
Binding of Src to its target proteins results in phosphorylation on putative tyrosine residues. Using a phosphotyrosine-specific antibody, 4G10, we performed Western blot on HEK293 cell samples transfected with HCN4 only, HCN4+Src529, and HCN4+Src296 (Fig. 6). At the calculated size of the HCN4 channel protein (129 kDa), a clear band is revealed for HCN4, a much stronger band for HCN4+Src529, and a much weaker band for HCN4+Src296. This result suggests that HCN4 channels are indeed tyrosine phosphorylated by Src529 in HEK293 cells.
FIGURE 6.
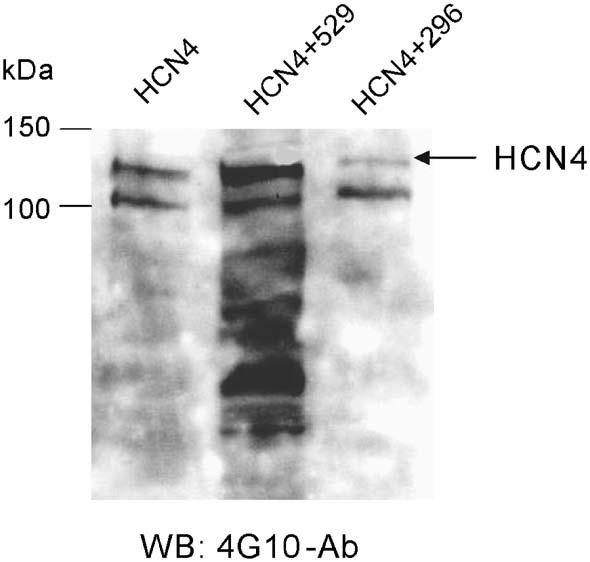
Tyrosine phosphorylation of HCN4 channels. Phosphorylated tyrosine signals in HEK cells transfected with HCN4, HCN4+Src529, and HCN4+Src296 were detected in Western blotting using a phosphotyrosine specific antibody, 4G10.
Interaction of Src With HCN4 Channels in Cardiac Cells
To extend the study of HCN4 channel modulation by Src to cardiac myocytes, we performed Western blot and coimmunoprecipitation experiments in adult rat ventricle. Using specific Src and HCN4 antibodies, weak but clear signals can be detected (Fig. 7A). The Src and HCN4 protein levels are estimated to be 69% ± 15% (n = 3) and 22% ± 3% (n = 3) of β-actin levels in rat ventricle, respectively (Fig. 7B).
When the rat ventricle sample was first immuno-precipitated with an HCN4 antibody followed by Western blotting using a Src antibody, a noticeable band can be visualized at the calculated size of Src protein (Fig. 7C). This result indicates that Src and HCN4 channel proteins can form a complex in rat ventricle.
DISCUSSION
Tyrosine phosphorylation has recently been recognized as a mechanism for regulation of membrane electrical properties via modulating the properties of ion channels. It has been well documented that Src tyrosine kinases Src, Fyn, and Yes can regulate the properties of potassium channels (Kv1.2,15 Kv1.3,14,16 Kv1.4,15 Kv1.5,17 Kv2.1,18 delayed rectifier,19,20 calcium-sensitive K channel21), sodium channels (NaV1.522), L-type calcium channels,23,24 chloride channels,25 cyclic nucleotide-gated channels,26,27 Na/K pump,28,29 and connexin 43.30 Thus, tyrosine phosphorylation can contribute to the control of membrane excitability and cell–cell communication.
The possible regulation of cardiac pacemaker activity by tyrosine kinases dates back to 1987, when EGF was shown to exert a positive chronotropic effect on cardiac cells in culture.4 The effect was later confirmed in rat heart.5 To explore the cellular mechanism of EGF action on pacemaker activity, we showed that EGF can increase the pacemaker current, if, in rabbit SA node cells.3 Furthermore, we showed that the enhancement of if by EGF could be prevented by genistein, a nonspecific tyrosine kinase inhibitor, suggesting that this effect was caused by an increased tyrosine kinase activity.3 Inhibition of if induced by reduced tyrosine kinase activity also occurs in rat ventricular myocytes.6 Interestingly, the effect of tyrosine kinase activity on if is tissue specific: In SA node, increased tyrosine kinase activity induced by EGF enhances if by increasing its whole cell conductance without affecting its voltage dependence.2 In the ventricle, decreased tyrosine kinase activity by genistein reduced if by decreasing maximal if current but also shifted the voltage dependence of if in the negative direction on the voltage axis.6 These different actions of tyrosine kinases on if are explained by tissue-specific expression and modulation of HCN channel isoforms.6,7
Furthermore, these actions of genistein were not caused by direct channel blockade as was previously suggested31 because they were not shared by an optical isomer of genistein (daidzein) that does not inhibit tyrosine kinases2,6 and another tyrosine kinase inhibitor, herbimycin, with a different mechanism of inhibition had a similar effect to genistein.2 Nevertheless, differences exist between our previous studies, which always showed effects on whole cell conductance2,3,6 and the results of our current study on the effects of Src, which only had an effect on gating properties. One possibility is that Src cannot phopshorylate all of the tyrosine phosphorylation sites on the channel protein and that the effects on conductance and gating properties are mediated through phosphorylation at different sites. The possibility of phosphorylating HCN4 channel protein at different tyrosine residues is also implicated in the effect of Src529 on the channel kinetics (Fig. 3). The slowest activation of the channel usually occurs at half-activation voltage. In the case of HCN4+Src529, the midpoint voltage is −80 mV, but the slowest activation appears at −55 mV.
Interaction of HCN4 With Src
A recent report has demonstrated that Src interacts with heterologously expressed HCN channels.9 In comparing the effects of coexpression of a dominant negative form of Src with HCN channels expressed alone, the authors concluded that the major biophysical effect of Src-mediated tyrosine phosphorylation is more rapid activation kinetics. They did not try to increase the activity of Src in their expression system because HEK293 cells are known to express appreciable Src protein. In the present work, we have used a constitutively active and a dominant negative form of Src proteins to study the tyrosine phosphorylation of HCN4 channels. We have shown that Src529 can shift the activation of HCN4 to more positive voltages (Fig. 2) and speed the activation kinetics (Fig. 3). We further demonstrated that the Src-induced changes in the properties of HCN4 channels expressed in HEK293 cells possibly result from direct binding of Src to the channel protein (Fig. 5). Moreover, association of Src protein with the HCN4 channel protein is also observed in rat ventricular myocytes (Fig. 7C).
Tyrosine Phosphorylation of HCN4 Channels
Although we do not have direct evidence showing that complex formation of HCN4 protein with Src results in phosphorylation on the putative tyrosine residue(s) of the channel, 3 pieces of data suggest HCN4 channels may be phosphorylated by Src after forming the HCN4 and Src protein complex.
First, the delay of if activation is remarkable in tissues outside the SA node (eg, Purkinje fibers/cells12 and ventricular myocytes6,13). Previous studies by us and others have shown that the duration of the delay can change in response to different phosphorylation states. Decreased protein kinase activity by H-7, a protein kinase inhibitor, was shown to increase the delay in if activation in canine Purkinje fibers.32 Increased phosphorylation by calyculin A, a nonspecific protein phosphatase inhibitor, decreases the delay in if activation in canine Purkinje and ventricular myocytes.6,13 In oocytes expressing HCN2 and HCN4 channels, we showed that genistein enhanced the initial delays in both HCN2 and HCN4 activation (see Figs. 2 and 3 in Reference 6). All 4 isoforms of HCN channels have exhibited delays in their activation when expressed in heterologous expression systems such as oocytes6,33 and mammalian cell lines.11,34 The delays in HCN2 and HCN4 current activation can be increased by genistein.6 Shortening and prolonging the delay in HCN4 current activation by Src529 and Src296, respectively, provides additional evidence for the possible phosphorylation of the pacemaker channel protein. In a recent report on Src regulation of HCN2,9 the authors also showed (although not mentioned in the text) significant delay in current activation of HCN2 (Figs. 1B, 1D, and 1E in Reference 9) and HCN4 (Fig. 7B in Reference 9) expressed in HEK cells and a prolonged delay in the presence of PP2, which inhibits Src kinase activity.
Second, the co-immunoprecipitation experiment revealed strong signals for HCN4 (Fig. 5E) and HCN4+Src529 (Figs. 5D, E), but an absent (Fig. 5D) or much reduced signal intensity (Fig. 5E) in HCN4+Src296 transfected cells. Src529 is a mutant that makes Src a constitutively active kinase. Src296 is a dominant negative mutant, which lacks the adenosine triphosphate-binding site for interaction with the endogenous activators of Src. Therefore, the results shown in Figures 5D and E support the notion that HCN4 channel protein is phosphorylated when forming a complex with Src529.
Finally, it is worth noting that delay in current activation is inversely related to the magnitude of hyperpolarization—less delay with steeper hyperpolarization.35 For the delay, hyperpolarization (which makes the membrane potential more negative) is equivalent to phosphorylation (which adds negative charge(s) to the target proteins).
Physiological Relevance of Src Modulation of HCN4
Among the 3 cardiac isoforms, HCN4 transcripts and proteins are expressed most abundantly in the sinus node,7,36 and least in the ventricle.7,37 When heterologously expressed, HCN4 exhibits more negative voltage dependence of activation and slower kinetics in comparison to sinus node if.3,6,11 Therefore, HCN4 channels must be modulated in vivo to make them a major contributor to sinus node if. Possible mechanisms of HCN4 modulation that have been investigated so far include: functional heteromerization,38 β-subunit modulation,39 and post-translational modulation.40,41
In this report, we provide evidence for a new mechanism of HCN4 channel modulation by Src tyrosine kinase. Src-mediated tyrosine phosphorylation of HCN4 channel protein shifts the channel activation to more positive potential and thus provides more depolarizing currents at diastolic membrane potentials (Fig. 2D). It also speeds the activation kinetics at certain voltages (Fig. 3A), and reduces the delay (Fig. 4E), making the properties of the functional HCN4 channels more similar to those of if channels in sinus node. Low expression of Src in ventricle coupled with low expression of HCN4 (compared to β-actin expression) makes the functional HCN4 channels behave more like ventricular if.
In addition, tyrosine phosphorylation by Src of the HCN4 channel may also shed light on the large difference in the voltage dependence of activation of if in newborn and adult ventricular myocytes. HCN4 is required in the genesis of cardiac pacemaker activity in early development.42 The threshold for activation of if in neonatal rat ventricle is around −70 mV but shifted to −113 mV in adult ventricle.43 In association with this age-dependent positive shift of if activation, there is a higher percentage of HCN4 mRNA expression in neonate than in adult rat ventricle.7 A strong correlation between HCN4 mRNA and protein expression has been recently observed,36 indicating the probability that there is more HCN4 channel protein in neonate than in adult rat ventricle. Interestingly, Src expression seems to be higher in neonatal than in adult rat ventricle28 (Fig. 7A). Thus, Src modulation of the HCN4 channel may represent one of the mechanisms that maintain the less negative voltage-dependent activation of if in sinus node and in neonatal ventricle to meet the various requirements of pacemaking activity in these 2 regions. Further structure-function studies of HCN4 channels will help reveal the structural basis of positive shift of HCN4 channels induced by tyrosine kinases.
CONCLUSION
In HEK293 cells and cardiac myocytes, we demonstrate the possible interaction between the HCN4 channel protein and Src tyrosine kinase. This interaction results in possible phosphorylation and alters the properties of HCN4 channels. It provides a novel mechanism for differential regulation of if channels, yielding a wide range of voltage-dependent activation in various cardiac regions.
Footnotes
Supported by a grant from American Heart Association (National Scientist Development Grant, 0030124N, HGY) and HL075023 from NHLBI (HGY) and was presented in abstract form at the meeting of the Biophysical Society in February 2005. I.S.C. is supported by HL28958 and HL67101 from the National Heart, Lung, and Blood Institute.
REFERENCES
- 1.Davis MJ, Wu X, Nurkiewicz TR, et al. Regulation of ion channels by protein tyrosine phosphorylation. Am J Physiol Heart Circ Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- 2.Wu JY, Cohen IS. Tyrosine kinase inhibition reduces if in rabbit SA myocytes. Pflügers Arch. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- 3.Wu JY, Yu H, Cohen IS. Epidermal growth factor increases if in rabbit SA node cells by activating a tyrosine kinase. Biochim Biophys Acta. 2000;1463:15–19. doi: 10.1016/s0005-2736(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 4.Nair BG, Rashed HM, Patel TB. Epidermal growth factor produces inotropic and chronotropic effects in rat hearts by increasing cyclic AMP accumulation. Growth Factors. 1993;8:41–48. doi: 10.3109/08977199309029133. [DOI] [PubMed] [Google Scholar]
- 5.Rabkin SW, Sunga P, Myrdal S. The effect of epidermal growth factor on chronotropic response in cardiac cells in culture. Biochem Biophys Res Commun. 1987;146:889–897. doi: 10.1016/0006-291x(87)90614-0. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Lu Z, Pan Z, et al. Tyrosine kinase inhibition differentially regulates heterologously expressed HCN channels. Pflügers Arch. 2004;447:392–400. doi: 10.1007/s00424-003-1204-y. [DOI] [PubMed] [Google Scholar]
- 7.Shi W, Wymore R, Yu H, et al. Distribution and prevalence of hyperpolarization-activated cation channel (HCN) mRNA expression in cardiac tissues. Circ Res. 1999;85:e1–e6. doi: 10.1161/01.res.85.1.e1. [DOI] [PubMed] [Google Scholar]
- 8.Santoro B, Grant SG, Bartsch D, et al. Interactive cloning with the SH3 domain of N-src identifies a new brain specific ion channel protein, with homology to eag and cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 1997;94:14815–14820. doi: 10.1073/pnas.94.26.14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zong X, Eckert C, Yuan H, et al. A novel mechanism of modulation of hyperpolarization-activaed cyclic nucleotide-gated channels by Src kinase. J Biol Chem. 2005;280:34224–34232. doi: 10.1074/jbc.M506544200. [DOI] [PubMed] [Google Scholar]
- 10.Arinsburg SA, Pastor D, Torres G, et al. Src changes gating of HCN4 channels through direct binding to the channel proteins. Biophys J. 2005;88:465A. doi: 10.1097/01.fjc.0000211740.47960.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig A, Zong X, Stieber J, et al. Two pacemaker channels from human heart with profoundly different activation kinetics. EMBO J. 1999;18:2323–2329. doi: 10.1093/emboj/18.9.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Chang F, Cohen IS. Phosphatase inhibition by calyculin A increases if in canine Purkinje fibers and myocytes. Pflugers Arch. 1993;422:614–616. doi: 10.1007/BF00374010. [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Chang F, Cohen IS. Pacemaker current If in adult canine cardiac ventricular myocytes. J Physiol. 1995;485:469–483. doi: 10.1113/jphysiol.1995.sp020743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitabach MN, Llamas DA, Araneda RC, et al. A mechanism for combinatorial regulation of electrical activity: potassium channel subunits capable of functioning as Src homology 3-dependent adaptors. Proc Natl Acad Sci U S A. 2001;98:705–710. doi: 10.1073/pnas.031446198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowlby MR, Fadool DA, Holmes TC, et al. Modulation of the Kv1.3 potassium channel by receptor tyrosine kinases. J Gen Physiol. 1997;110:601–610. doi: 10.1085/jgp.110.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes TC, Fadool DA, Ren R, et al. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 18.Tong Y, Brandt GS, Li M, et al. Tyrosine decaging leads to substantial membrane trafficking during modulation of an inward rectifier potassium channel. J Gen Physiol. 2001;117:103–118. doi: 10.1085/jgp.117.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X-Y, Morielli AD, Peralta EG. Tyrsoine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 20.Sobko A, Peretz A, Attali B. Constitutive activation of delayed-rectifier potassium channels by a Src family tyrosine kinase in Schwann cells. EMBO J. 1998;17:4723–4734. doi: 10.1093/emboj/17.16.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ling S, Woronuk G, Sy L, et al. Enhanced activity of a large conductance, calcium-sensitive K+ channel in the presence of Src tyrosine kinase. J Biol Chem. 2000;275:30683–30689. doi: 10.1074/jbc.M004292200. [DOI] [PubMed] [Google Scholar]
- 22.Ahern CA, Zhang J-F, Wookalis MJ, et al. Modulation of the cardiac sodium channel NaV1.5 by Fyn, a Src family tyrosine kinase. Circ Res. 2005;96:991–998. doi: 10.1161/01.RES.0000166324.00524.dd. [DOI] [PubMed] [Google Scholar]
- 23.Bence-Hanulec KK, Marshall J, Blair LAC. Potentiation of neuronal L calcium channels by IGF-1 requires phosphorylation of the α1 subunit on a specific tyrosine residue. Neuron. 2000;27:121–131. doi: 10.1016/s0896-6273(00)00014-3. [DOI] [PubMed] [Google Scholar]
- 24.Schröder F, Klein G, Frank T, et al. Src family tyrosine kinases inhibit single L-type: Ca2+ channel activity in human atrial myocytes. J Mol Cell Cardiol. 2004;37:735–745. doi: 10.1016/j.yjmcc.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Browe DM, Baumgarten CM. Stretch of b1 integrin activates an outwardly rectifying chloride current via FAK and Src in rabbit ventricular myocytes. J Gen Physiol. 2003;122:689–702. doi: 10.1085/jgp.200308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molokanova E, Trivedi B, Savchenko A, et al. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci. 1997;17:9068–9076. doi: 10.1523/JNEUROSCI.17-23-09068.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molokanova E, Savchenko A, Kramer RH. Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. J Gen Physiol. 1999;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 29.Haas M, Wang H, Tian J, et al. Src-mediated interceptor cross-talk between the Na/K-ATPase and the EGF receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–18702. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 30.Kanemitsu MY, Loo LWM, Simon S, et al. Tyrosine phosphorylation of connexin 43 by v-Src is mediated by SH2 and SH3 domain interactions. J Biol Chem. 1997;272:22824–22831. doi: 10.1074/jbc.272.36.22824. [DOI] [PubMed] [Google Scholar]
- 31.Shibata S, Ono K, Iijima T. Inhibition by genistein of the hyperpolarization-activated cation current in porcine sino-atrial node cells. Br J Pharmacol. 1999;128:1284–1290. doi: 10.1038/sj.bjp.0702903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang F, Cohen IS, DiFrancesco D, et al. Effects of protein kinase inhibitors on canine Purkinje fibre pacemaker depolarization and the pacemaker current if. J Physiol. 1991;440:367–384. doi: 10.1113/jphysiol.1991.sp018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Wu J, Potapova I, et al. MinK-related peptide 1: a beta subunit for the HCN ion channel family, enhances expression and speeds kinetics. Circ Res. 2001;88:e84–e87. doi: 10.1161/hh1201.093511. [DOI] [PubMed] [Google Scholar]
- 34.Mistrík P, Mader R, Michalakis S, et al. The murine HCN3 gene encodes a hyperpolarization-activated cation channels with slow kinetics and unique response to cyclic nucleotides. J Biol Chem. 2005;280:27056–27061. doi: 10.1074/jbc.M502696200. [DOI] [PubMed] [Google Scholar]
- 35.DiFrancesco D, Ferroni A. Delayed activation of the cardiac pacemaker current and its dependence on conditioning prehyperpolarizations. Pflügers Arch. 1983;396:265–267. doi: 10.1007/BF00587866. [DOI] [PubMed] [Google Scholar]
- 36.Brioschi C, Dobrzynski H, Terragni B, et al. Molecular Localization of HCN4 in Rabbit Sinoatrial Node [abstract]. 4th Mammalian Myocardium Meeting program. Bristol University; Bristol, UK: 2005. p. 2. [Google Scholar]
- 37.Han W, Bao W, Wang Z, et al. Comparison of ion-channel subunit expression in canine cardiac Purkinje fibers and ventricular muscle. Circ Res. 2002;91:790–797. doi: 10.1161/01.res.0000039534.18114.d9. [DOI] [PubMed] [Google Scholar]
- 38.Altomare C, Terragni B, Brioschi C, et al. Heteromeric HCN1–HCN4 channels: a comparison with native pacemaker channels from the rabbit sinoatrial node. J. Physiol. 2003;549:347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decher N, Bundis F, Vajna R, et al. KCNE2 modulates current amplitudes and activation kinetics of HCN4: influence of KCNE family members on HCN4 currents. Pflügers Arch. 2003;446:633–640. doi: 10.1007/s00424-003-1127-7. [DOI] [PubMed] [Google Scholar]
- 40.Barbuti A, Gravante B, Riolfo M, et al. Localization of pacemaker channels in lipid rafts regulates channel kinetics. Circ Res. 2004;94:1325–1331. doi: 10.1161/01.RES.0000127621.54132.AE. [DOI] [PubMed] [Google Scholar]
- 41.Much B, Wahl-Schott C, Zong X, et al. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Frigola C, Shi Y, Evans SM. Expression of the hyperpolarization-activated cyclic nucleotide-gated cation channel HCN4 during mouse heart development. Gene Expression Patterns. 2003;3:777–783. doi: 10.1016/s1567-133x(03)00125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson RB, Yu H, Chang F, et al. Developmental change in the voltage-dependence of the pacemaker current if in rat ventricle cells. Pflügers Arch. 1997;433:533–535. doi: 10.1007/s004240050309. [DOI] [PubMed] [Google Scholar]