Abstract
Biomaterial-associated infections (BAI) are the major cause of failure of indwelling medical devices and are predominantly caused by staphylococci, especially Staphylococcus epidermidis. We investigated the in vitro microbicidal activity of the synthetic antimicrobial peptide bactericidal peptide 2 (BP2) and its efficacy in a murine model of S. epidermidis BAI. BP2 showed potent microbicidal activity at micromolar concentrations against a broad spectrum of microorganisms, including antibiotic-resistant bacteria. The staphylocidal activity of BP2 was not affected by physiological salt concentrations and was only slightly affected by the presence of human plasma. In the BAI model, injection of BP2 (5 mg/kg of body weight) 1 h after challenge with S. epidermidis resulted in an 80% reduction in the number of culture-positive implants and a 100-fold reduction in survival of S. epidermidis in peri-implant tissue at 24 h postchallenge. When BP2 was injected along implants 3 h prior to bacterial challenge, the median numbers of CFU cultured from biomaterial implants and peri-implant tissue were reduced by 85% and 90%, respectively. In conclusion, BP2 has potent, broad-spectrum in vitro microbicidal activity and showed potent in vivo activity in a murine model of S. epidermidis biomaterial-associated infection.
Biomaterial-associated infections (BAI) are the major cause of failure of indwelling medical devices (16, 39, 48). Frequencies of BAI vary from 0.1 to 1% for intraocular lenses to >20% for catheters used for chronic ambulatory peritoneal dialysis (39, 48). The presence of indwelling medical devices is a major risk factor for nosocomial infections (38). Infections of catheters often result in prolonged hospitalization, the need for surgery with removal of the device, and even death (5, 25). In the United States alone, approximately 2 million nosocomial biomaterial-associated infections cost nearly $11 billion annually (38).
Coagulase-negative staphylococci, particularly Staphylococcus epidermidis, are the major cause of BAI (16, 39, 48).
The pathogenesis of BAI is a complex, multifactorial process influenced by physicochemical properties of the biomaterial, adherence of host proteins, bacterial virulence factors, and alterations of the host defense (7, 48). The production of extracellular polysaccharides (slime) leads to the formation of a biofilm by bacteria adherent to the biomaterial surface (18, 33, 43). This biofilm is believed to facilitate bacterial persistence by impairing host defenses and impeding antibiotic penetration (13, 19). Established implant infections are highly persistent despite antibiotic treatment and often necessitate removal of the implant (16, 39). The persistence of S. epidermidis in peri-implant tissue has also been demonstrated (7, 47) and has been associated with abscess formation (7).
To prevent biomaterial-associated infections, antibiotic prophylaxis is recommended for various medical conditions requiring biomaterial implants (15, 23), but a major concern is the development of resistance (32, 35, 37). Therefore, new strategies for prophylaxis or treatment of biomaterial-associated infections are required. A promising class of antimicrobial agents are the cationic antimicrobial peptides (AMPs). These molecules are an important first line of defense against microorganisms and have been identified in all living organisms studied, including plants, insects, humans, and other mammals (26). A general characteristic of AMPs is an amphipathic structure with both hydrophobic and cationic domains (22, 40, 44). Most AMPs are believed to directly target the negatively charged microbial cell membrane and mediate killing by membrane disruption or pore formation (40, 49, 52), although for some AMPs an intracellular target has been proposed (10, 29, 42).
Bactericidal peptide 2 (BP2) is a small synthetic antimicrobial peptide designed based on lipopolysaccharide-binding domains of various naturally occurring proteins and has the structural elements for an amphipathic conformation (2). The aim of the present study was to characterize the antimicrobial spectrum of BP2 in vitro and to assess its potential for prevention or treatment of experimental murine biomaterial-associated S. epidermidis infection.
MATERIALS AND METHODS
Peptides.
BP2 (GKWKLFKKAFKKFLKILAC) was synthesized at Pepscan Systems (Lelystad, The Netherlands), using solid-phase 9-fluorenylmethoxycarbonyl chemistry with a free amine at the N terminus and a free amide at the C terminus. BP2 was purified by high-performance liquid chromatography, and its purity (>95%) and mass were confirmed by ion-spray mass spectrometry. The lack of disulfide formation between free cysteines of BP2 was confirmed by quantitative time-of-flight-mass spectrometry analysis. Human neutrophil defensins isolated from neutrophil granules as a mixture of HNP-1, -2, and -3 (46) and synthetic cathelicidin LL-37 (1) were a kind gift of P. S. Hiemstra (Leiden).
Microorganisms.
The microbicidal activity of BP2 was assessed against Bacillus subtilis ATCC 6633, Escherichia coli ML-35 (30), Staphylococcus aureus 42D (51), Staphylococcus epidermidis RP62a (ATCC 35984), Streptococcus oralis J30 (17), and Burkholderia cepacia ATCC 25416 and against clinical isolates of Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Bacteroides fragilis, Brucella abortus, enteropathogenic E. coli, and Clostridium perfringens, methicillin-susceptible and -resistant isolates of S. epidermidis and S. aureus, and vancomycin-susceptible and -resistant isolates of Enterococcus faecium. Candida albicans and Cryptococcus neoformans clinical isolates were used to test fungicidal activity.
The oxacillin susceptibility of S. aureus and S. epidermidis isolates and the vancomycin susceptibility of E. faecium isolates were determined by Etest (AB Biodisk, Solna, Sweden) according to the manufacturer's instructions.
Animals.
Specific-pathogen-free C57BL/6 mice (Harlan, Horst, The Netherlands), aged 6 to 8 weeks old and weighing 15 to 20 g, were used. Mice were housed in individual cages in a pathogen-free environment and provided with sterile food and water. The Animal Care and Use Committee of the University of Amsterdam approved all experiments.
Plasma.
Fresh blood was obtained from healthy volunteers, using 8-ml citrate-containing Vacutainer tubes (BD Vacutainer Systems, Franklin Lakes, NJ). Plasma was obtained after centrifugation at 800 × g for 20 min.
Microbicidal assays.
The microbicidal activity of BP2 was quantified with a minimal bactericidal/fungicidal concentration (MBC/MFC) assay, essentially as described by Harwig et al. (24). This assay is often used to assess the microbicidal activities of antimicrobial peptides (6, 34). We chose this method rather than the CLSI (formerly NCCLS) standard MBC assay for antibiotic activity testing since the CLSI method uses Mueller-Hinton medium, in which cationic peptides may aggregate (45). Overnight cultures in Trypticase soy broth (TSB; Difco, Detroit, MI) were diluted 100-fold in fresh TSB and cultured for 3 h at 37°C. B. abortus was grown in the presence of 5% CO2, C. perfringens and B. fragilis were grown anaerobically, and all other bacteria were cultured aerobically. Fungi were cultured for 48 h at 30°C in 0.7% (wt/vol) yeast nitrogen base (Difco) supplemented with 0.15% (wt/vol) l-asparagine (Merck, Darmstadt, Germany) and 1% (wt/vol) glucose (Merck). Bacteria and fungi were washed twice with 10 mM phosphate buffer, pH 7.0, plus 0.06% (wt/vol) TSB, the optical density at 620 nm was measured, and the bacteria and fungi were diluted to 2 × 106 CFU/ml in 10 mM phosphate buffer, pH 7.0, plus 0.06% (wt/vol) TSB (incubation buffer), based on an established relationship between optical density and the number of CFU. Twenty-five-microliter aliquots of twofold serially diluted BP2 were prepared in a low-protein-binding polypropylene microtiter plate (Costar, Corning, NY). To each of the wells, 25 μl bacteria or fungi was added. After 2 h of incubation on a rotary shaker at 150 rpm at 37°C (bacteria) or 30°C (fungi), duplicate 10-μl aliquots were plated on blood agar plates. The plates were inspected for growth after 24 h (bacteria) or 48 h (fungi). The MBC/MFC was defined as the lowest concentration of BP2 at which <0.1% of an inoculum of 106 CFU/ml survived after 2 h of exposure. At least two independent experiments were performed, with each sample plated out in duplicate. When the MBCs of the independent experiments differed, the experiments were repeated, and the median value for all four independent assays was defined as the MBC.
To study killing kinetics, inocula and BP2 were prepared as described above. At sampling time points, 10-μl samples were mixed with 20-μl aliquots of 0.05% (wt/vol) of the polyanionic agent sodium polyanetholesulfonate, which instantly neutralized BP2. Sodium polyanetholesulfonate did not affect the viability of the microorganisms tested. The number of viable microorganisms was assessed by quantitative culture of serial 10-fold dilutions in incubation buffer.
To determine the influence of human plasma on BP2 activity, bacterial suspensions were diluted to 107 CFU/ml in incubation buffer, and 10-μl aliquots were added to 80 μl of various concentrations of human plasma prepared in incubation buffer. Ten-microliter aliquots of BP2 were added, and the killing kinetics was determined as described above.
Experimental biomaterial-associated infection model. (i) Biomaterial implants.
In a laminar-flow cabinet, 1-centimeter-long segments of polyvinylpyrrolidone-grafted silicon elastomer catheters (SEpvp; Bioglide Medtronic PS Medical, Goleta, CA), with an external diameter of 2.5 mm and a wall thickness of 0.6 mm, were cut and stored in sterile petri dishes.
(ii) S. epidermidis RP62a inoculum.
S. epidermidis strain RP62a (ATCC 35984) was used as the challenge strain. This strain is capable of producing slime, determined as described by Christensen et al. (11). A log-phase culture of S. epidermidis RP62a in TSB was centrifuged at 3,000 × g for 10 min, and the pelleted bacteria were washed twice with 50 ml of sterile pyrogen-free 0.9% NaCl (saline; Baxter, Lessines, Belgium). After the final centrifugation, the pellet was resuspended in saline, the optical density at 620 nm was measured, and the suspension was diluted to 4 × 107 CFU/ml in saline. Twenty-five microliters of this suspension (106 CFU) was used as the inoculum.
(iii) Subcutaneous biomaterial implantation and administration of the bacterial inoculum.
Mice were anesthetized by intraperitoneal injection with FFM mix (1 ml of fentanyl citrate [Hypnorm; also called Fluanisone], 1 ml of midazolam, and 2 ml of distilled water; 0.07 ml mix per 10 g of body weight) and were placed in a laminar-flow cabinet. The backs of the mice were shaved and disinfected with 70% ethanol. On each side, an incision of 0.3 cm was made 1 cm lateral to the spine. Subsequently, 1-cm-long SEpvp segments were implanted subcutaneously with minimal tissue damage, using a transponder. The incisions were closed with a single 0/6 vicryl stitch (9).
In the treatment protocol, the S. epidermidis inoculum was injected along the implants immediately following surgery. One hour later, BP2 (5 mg/kg in 50 μl) was injected along the implants. In the prophylaxis protocol, BP2 (5 mg/kg in 50 μl) was injected along the implants immediately after implantation, and 3 h later the inoculum (25 μl) was injected along the implants. All injections were performed with a highly accurate repetitive injector (Stepper model 4001-025; Tridak Division, Brookfield, Conn.).
(iv) Sample collection.
One day after challenge, mice were anesthetized with FFM mix and subsequently sacrificed by cardiac puncture. Standardized biopsies (12 mm in diameter) were taken from the implantation sites as described previously (8). Each biopsy included skin, subcutaneous tissue, and the implant.
The biomaterial implants were separated from the tissue, rinsed twice with 10 ml pyrogen-free saline, and placed in a sterile tube containing 1 ml of 0.9% NaCl. The tissue samples were placed in a tube and weighed, and a volume of saline corresponding to four times the weight was added. The weight of the tissue samples varied between 60 and 250 mg.
(v) Quantitative culture of biomaterial implants.
Tubes containing the implants in 0.5 ml of NaCl were sonicated for 30 s in a water bath sonicator (Elma Transsonic T460, 35 kHz; Elma, Singen, Germany) to dislodge adherent bacteria. The number of viable S. epidermidis cells was assessed by quantitative culture of serial 10-fold dilutions of the sonicate. In addition, the sonicated implants were cultured in 80 ml of modified thioglycolate broth (TB), consisting of 3% (wt/vol) thioglycolate containing 0.03% (wt/vol) polyanetholesulfonic acid, 1 M NaOH, and 0.5% Tween 80, for 72 h at 37°C. Antibiotic susceptibilities, as reported previously (9), were used to confirm the identities of bacteria cultured from biomaterial implants and tissue homogenates. A value of 5 CFU per 1-cm biomaterial implant was assigned to implants for which the sonicates did not yield growth on blood agar plates but were culture positive in broth. For representation on a logarithmic scale, a value of 1 CFU per biomaterial implant was assigned when no growth occurred at all.
(vi) Quantitative culture of homogenates.
Tubes containing the tissue samples in saline were homogenized on ice with a tissue homogenizer (Tissue Tearor model 985-370; Biospec Products, Bartlesville, OK). Before each homogenization, the homogenizer was carefully cleaned, disinfected with 0.4% (wt/vol) sodium hypochlorite followed by 70% alcohol, and rinsed with sterile water and saline. Homogenates were 10-fold serially diluted and cultured as described for the sonicates from implants. In addition, 1/10 of each homogenate was cultured in 80 ml TB for 72 h at 37°C. The number of cultured S. epidermidis RP62a cells is expressed in CFU per biopsy. A value of 10 CFU was assigned when S. epidermidis was cultured in TB but not on blood agar plates. For representation on a logarithmic scale, a value of 1 CFU per biopsy was assigned when no growth occurred at all.
Statistical analysis.
Numbers of bacteria cultured from biopsies or implants are expressed as median values. Two-sample comparisons were made using a two-tailed Mann-Whitney rank sum test. The significance of differences between the frequencies of categorical variables was determined using Fisher's exact test. For all tests, P values of <0.05 were considered significant.
RESULTS
BP2 has broad-spectrum microbicidal activity in vitro.
The microbicidal activity of BP2 in vitro was tested against a wide panel of gram-positive and -negative bacteria and fungi, using an MBC/MFC assay. BP2 had potent and broad-spectrum microbicidal activity, similar to that of the highly potent LL-37 and approximately fourfold higher than that of HNP1-3, with MBC/MFC values in the low micromolar range for most test microorganisms (Table 1). Only Proteus mirabilis and Burkholderia cepacia, known to be resistant to other cationic antimicrobial peptides, were not killed by 30 μM BP2. Staphylococcus epidermidis RP62a, the strain used in our experimental biomaterial-associated infection model, was highly susceptible to BP2, with an MBC of 1 μM. In addition, antibiotic-resistant strains of S. epidermidis, Staphylococcus aureus, and Enterococcus faecium had MBCs for BP2 similar to those of antibiotic-sensitive strains (Table 1).
TABLE 1.
Microbicidal spectrum of BP2
| Microorganism | MBC/MFCa (μM)
|
||
|---|---|---|---|
| BP2 | LL-37 | HNP1-3 | |
| Gram-negative bacteria | |||
| Enteropathogenic Escherichia coli | 4 | ||
| Escherichia coli ML-35 | 2 | 2 | >15 |
| Pseudomonas aeruginosa | 8 | ||
| Klebsiella pneumoniae | 4 | ||
| Proteus mirabilis | >30 | ||
| Burkholderia cepacia | >30 | ||
| Bacteroides fragilis | 4 | ||
| Brucella abortus | 4 | ||
| Gram-positive bacteria | |||
| Bacillus subtilis ATCC 6633 | 1 | 0.5 | 4 |
| Clostridium perfringens | 1 | ||
| Staphylococcus aureus 42D | 2 | 1 | >15 |
| Streptococcus oralis J30 | 4 | ||
| Enterococcus faecium | 4 | ||
| Staphylococcus epidermidis RP62a | 1 | 2 | >15 |
| Methicillin-sensitive S. aureus | 2, 2, 2 | ||
| Methicillin-resistant S. aureus | 2, 2, 2, 2, 8 | ||
| Methicillin-sensitive S. epidermidis | 1, 2, 8 | ||
| Methicillin-resistant S. epidermidis | 1, 2, 2, 4, 4, 4, 4 | ||
| Vancomycin-sensitive E. faecium | 4, 8, 8 | ||
| Vancomycin-resistant E. faecium | 4, 4, 8, 8, 8 | ||
| Fungi | |||
| Candida albicans | 15 | ||
| Cryptococcus neoformans | 4 | 8 | >15 |
Defined as the lowest concentration of BP2 that killed 99.9% of an inoculum of 106 CFU/ml within 2 h. A 1 μM concentration of BP2 corresponds to 2.3 μg/ml. Multiple values are for different clinical isolates.
Kinetics of microbicidal activity of BP2.
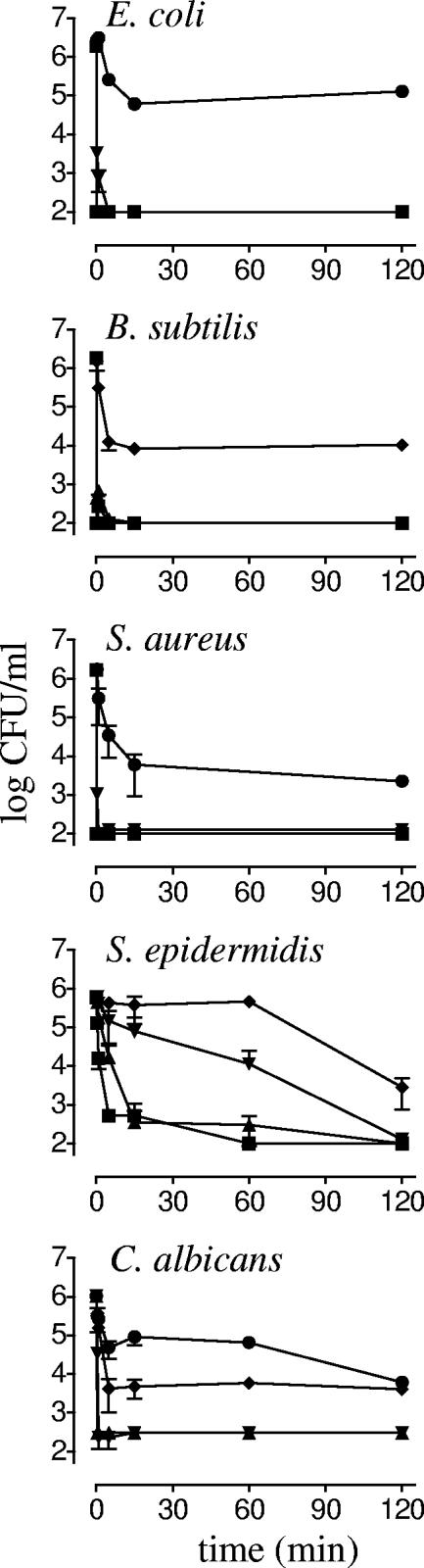
The kinetics of the microbicidal activity of BP2 for Escherichia coli, Bacillus subtilis, S. aureus, S. epidermidis, and Candida albicans at concentrations between 0.25 and 4 times the MBC/MFC were determined (Fig. 1). At or above the MBC/MFC, inocula of E. coli, B. subtilis, S. aureus, and C. albicans were completely killed within 1 min. Incubation with 0.25 to 0.5 times the MBC resulted in about 100-fold reductions in numbers of CFU within 15 min, after which no additional microbicidal activity was detected. For S. epidermidis, concentration-dependent kinetics of killing was observed: at 4 times the MBC, the entire inoculum was killed within 5 min, while at the MBC, this required 2 h of incubation.
FIG. 1.
Kinetics of killing of microorganisms by BP2. E. coli ML-35, B. subtilis ATCC 6633, S. aureus 42D, S. epidermidis RP62a, and a C. albicans clinical isolate were exposed to 0.25 (•), 0.5 (⧫), 1 (▾), 2 (▴), and 4 (▪) times the MBC/MFC of BP2. Mean survival (log CFU/ml) was assessed at the indicated time points.
Influence of physiological concentrations of NaCl and of plasma on bactericidal activity of BP2.
To assess the potential of BP2 for in vivo application, bactericidal activity was determined in the presence of physiological concentrations of NaCl. The MBCs of BP2 for S. epidermidis in 10 mM phosphate buffer and in phosphate-buffered saline were both 2 μM, indicating that a physiological salt concentration did not reduce the bactericidal activity of BP2. Similarly, the MBC of BP2 for S. aureus was 1 μM in 10 mM phosphate buffer as well as in phosphate-buffered saline.
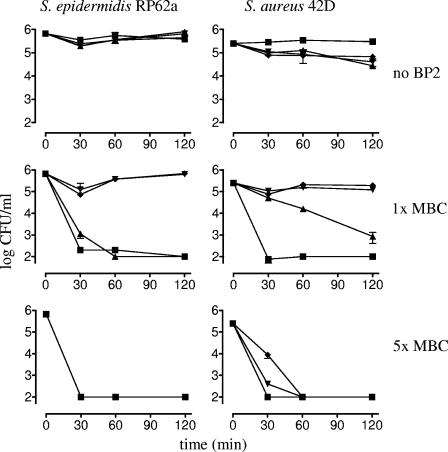
The bactericidal activity of BP2 was also assessed in the presence of human plasma. Killing of S. epidermidis by BP2 at its MBC was inhibited by ≥50% plasma (Fig. 2). However, when incubated with BP2 at a concentration of 5 times the MBC, the entire S. epidermidis inoculum was killed within 30 min, irrespective of the concentration of plasma (up to 75%) (Fig. 2). At the MBC, the bactericidal activity of BP2 against S. aureus was inhibited by 25% plasma (Fig. 2), but at 5 times the MBC, S. aureus was completely killed within 1 h at all concentrations of plasma tested (Fig. 2). Thus, at 5 times the MBC, staphylococci were rapidly killed by BP2 despite the presence of up to 75% plasma.
FIG. 2.
Influence of plasma on bactericidal activity of BP2. S. epidermidis RP62a and S. aureus 42D were incubated with various concentrations of BP2 in the absence of plasma (▪) or with 25% (▴), 50% (▾), or 75% (⧫) freshly prepared human plasma. The mean (± standard deviation) survival (log CFU/ml) of S. epidermidis RP62a and S. aureus 42D incubated without BP2 or with 1 times the MBC or 5 times the MBC of BP2 is shown.
Treatment of experimental biomaterial-associated S. epidermidis infection of mice with BP2.
The in vivo efficacy of BP2 was first tested in a treatment model of biomaterial-associated infection. BP2 (5 mg/kg) or saline was injected along subcutaneous implants 1 h after challenge with 106 CFU of S. epidermidis RP62a. Mice were sacrificed after 24 h, and bacterial survival on implants and in peri-implant tissues was assessed.
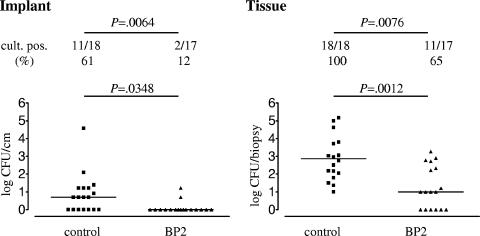
For the saline control group, 11 (61%) of 18 biomaterial implants were culture positive for S. epidermidis (Fig. 3). Treatment with BP2 reduced the number of culture-positive implants to 2 (12%) of 17 (Fig. 3) (P = 0.0064). The implants from the BP2-treated mice yielded significantly smaller numbers of CFU than those from the control mice (P = 0.0348).
FIG. 3.
Efficacy of BP2 in treatment of biomaterial-associated S. epidermidis infection. One hour after implantation of biomaterials and challenge with 106 CFU of S. epidermidis RP62a, mice received an injection of 50 μl saline or BP2 (5 mg/kg) subcutaneously along each implant. Mice were sacrificed after 24 h, and numbers of CFU adherent to the implants and present in peri-implant tissue were assessed. Two-sample comparisons were made using a two-tailed Mann-Whitney rank sum test. The significance of differences between the frequencies of categorical variables was determined using Fisher's exact test.
For the control group, bacteria were recovered from all peri-implant tissues, while for the BP2-treated group no bacteria were recovered from 6 (35%) of 17 biopsies (Fig. 3) (P = 0.0076). The median numbers of CFU cultured from peri-implant tissues of BP2-treated mice and saline controls were 10 and 760 CFU/biopsy, respectively (Fig. 3) (P = 0.0012).
Thus, BP2 effectively reduced the number of culture-positive implants and the number of CFU of S. epidermidis RP62a cultured from peri-implant tissue in the treatment model of biomaterial-associated S. epidermidis infection.
Prophylaxis with BP2 against experimental biomaterial-associated S. epidermidis infection in mice.
To assess the efficacy of BP2 in a prophylaxis regimen, BP2 was injected along subcutaneous implants 3 h prior to a challenge with 106 CFU of S. epidermidis RP62a. As in the treatment model, mice were sacrificed 24 h after injection, and bacterial survival was assessed.
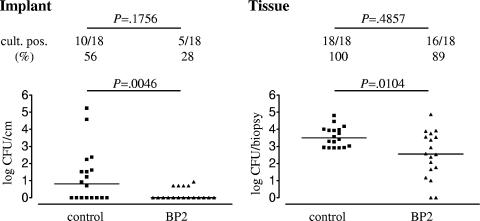
For the saline control group, 10 (56%) of 18 implants were culture positive for S. epidermidis (Fig. 4), whereas 5 (28%) of 18 implants were culture positive for the group that received BP2 prophylaxis (not significant [P = 0.1756]). The implants from the BP2-treated mice yielded significantly smaller numbers of CFU than those from the control mice (Fig. 4) (P = 0.0046).
FIG. 4.
Efficacy of BP2 in prophylaxis against biomaterial-associated S. epidermidis RP62a infection. SEpvp biomaterial segments were implanted subcutaneously in C57BL/6 mice, and BP2 (5 mg/kg) or saline was injected along the implanted segments 3 h prior to challenge with 106 CFU of S. epidermidis RP62a. Mice were sacrificed after 24 h, and numbers of CFU adherent to the implants and present in peri-implant tissue were assessed. Two-sample comparisons were made using a two-tailed Mann-Whitney rank sum test. The significance of differences between the frequencies of categorical variables was determined using Fisher's exact test.
For the control group, bacteria were recovered from all peri-implant tissues, and for the BP2-treated group, 16 (89%) of 18 samples were culture positive (Fig. 4) (not significant [P = 0.4857]). The median survival of S. epidermidis in peri-implant tissue was reduced from 3.2 × 103 CFU/biopsy for the saline controls to 363 CFU/biopsy after prophylactic administration of BP2 (P = 0.0104).
DISCUSSION
Biomaterial-associated infections, which are most frequently caused by S. epidermidis, are the major cause of failure of indwelling medical devices. Antibiotic strategies for treatment or prophylaxis of BAI often fail. In addition, frequent use of antibiotics increases the risk for the development of bacterial resistance. Therefore, the development of novel therapeutic agents to combat BAI is required. Antimicrobial peptides, which have potent broad-spectrum microbicidal activities and a low risk for the development of resistance, are promising candidates. In the present study, we demonstrated that BP2 has potent broad-spectrum microbicidal activity in vitro, including activity against antibiotic-resistant staphylococci and enterococci. We show that BP2 is more active than HNP1-3 and as active as LL-37, a highly potent human antimicrobial peptide. Unlike LL-37 (4) and HNP1-3 (36, 50), the microbicidal activity of BP2 is not inhibited by physiological salt concentrations, stressing the potential of BP2. In a murine model of biomaterial-associated S. epidermidis infections, BP2 proved to be an interesting candidate for therapeutic applications. A single dose of BP2 injected locally 1 h after or 3 h prior to bacterial challenge significantly reduced adherence of S. epidermidis to implanted biomaterials, as well as peri-implant tissue colonization.
In the in vitro assay, all gram-positive bacteria tested were highly susceptible to BP2, with MBCs of ≤4 μM, and most gram-negative bacteria were also susceptible, with MBCs of ≤8 μM. P. mirabilis and B. cepacia, which are known to be inherently resistant to AMPs (14, 41), were indeed also resistant to BP2 up to a concentration of at least 30 μM. Antibiotic-resistant clinical isolates of S. aureus, S. epidermidis, and E. faecium strains were as susceptible to BP2 as antibiotic-sensitive isolates, offering the prospect of BP2 as a novel antimicrobial agent. In addition, BP2 almost instantaneously killed E. coli, B. subtilis, S. aureus, S. epidermidis, and C. albicans. Moreover, the microbicidal activity of BP2 was not reduced at physiological salt concentrations in vitro, and its activity was only slightly affected by human plasma. Based on these promising characteristics, we assessed the potential for the application of BP2 in vivo, using a murine model of biomaterial-associated infection.
We used an established murine model of biomaterial-associated infection in which the injection of 106 CFU of S. epidermidis along SEpvp segments implanted subcutaneously in C57BL/6 mice causes the persistence of >3,000 CFU of S. epidermidis in peri-implant tissue and small numbers of S. epidermidis cells adhering to biomaterial implants for at least 2 days, with the persistence of small numbers of bacteria in the tissue for up to 60 days (9). Recent studies at our laboratory with different strains of S. epidermidis, different biomaterials, and different strains of mice have shown that the peri-implant tissue is the predominant site of S. epidermidis infection in all cases (C. A. N. Broekhuizen, personal communication). This indicates that survival of bacteria in peri-implant tissue may also be an important determinant in the pathogenesis of BAI.
Administration of BP2 1 h after challenge with S. epidermidis resulted in an almost 100-fold reduction in the median number of CFU in peri-implant tissues compared to those for the control group, demonstrating the potent bactericidal activity of BP2 in vivo. In addition, nearly all biomaterial implants (88%) were completely culture negative 24 h after BP2 treatment, whereas for the control group the majority (61%) were still culture positive.
Since little is known about the pharmacokinetics of BP2, we wondered whether BP2 would also be effective when administered prior to bacterial challenge. When BP2 was injected 3 h prior to challenge with S. epidermidis, most biomaterial implants (72%) were completely culture negative 24 h later, whereas 56% of the control implants still contained viable adherent bacteria. In addition, an approximately 10-fold reduction in the median number of CFU in peri-implant tissues was achieved. Although it was slightly less effective than in the treatment protocol, BP2 clearly had potent bactericidal activity when administered prophylactically several hours prior to bacterial challenge, indicating good peptide stability in vivo. We chose to administer BP2 at the site of infection as a strategy for the prevention or treatment of BAI, since these infections usually are caused by bacteria contaminating the implant during insertion or implantation. Since the tissue distribution of BP2 is as yet unknown, the potential of BP2 for other than local application remains to be determined.
AMPs are increasingly being tested for their clinical potential in various animal models and also in clinical trials (20, 21, 31, 53). In only a limited number of studies has the application of AMPs for prevention or treatment of BAI been assessed. Omiganan (MBI-226), a synthetic analogue of bovine neutrophil indolicidin (27), has advanced to clinical trials for the prevention of catheter-related bloodstream infections. Exit-site application of a 1% gel formulation of this antimicrobial peptide significantly reduced catheter colonization and catheter-related local infections in a phase IIIa study (21). A confirmatory phase IIIb study to assess whether omiganan can reduce the frequency of catheter-related bloodstream infections has been initiated (28). Prophylactic efficacies of the antimicrobial peptide temporin A (12) and of a derivative of dermaseptin (3) in a subcutaneous rat model have been reported. When Dacron grafts were presoaked in a solution containing 50 mg/liter of the dermaseptin derivative prior to infection with S. epidermidis or S. aureus, there was no evidence of bacterial colonization of grafts at 7 days postinfection, in contrast to >106 CFU/cm2 for untreated controls (3). In a similar experiment, presoaking with 10 mg/liter temporin A resulted in a >3-log reduction in bacterial colonization (12). Clearly, antimicrobial peptides have potential for therapeutic application in biomaterial-associated infections.
The present study demonstrated that the local injection of BP2 strongly reduced the survival of S. epidermidis in peri-implant tissue and the colonization of biomaterial implants. In addition, other applications of BP2 for prevention of BAI, such as coating of biomaterials or use in exit-site dressings, could be developed.
Acknowledgments
This work was supported by a SENTER grant (TSGE2055) from the Dutch Ministry of Economic Affairs.
We thank Leonie de Boer, Laura Boszhard, Corine Broekhuizen, and Bert van Urk for assistance with animal experimentation.
Footnotes
Published ahead of print on 25 September 2006.
REFERENCES
- 1.Aarbiou, J., G. S. Tjabringa, R. M. Verhoosel, D. K. Ninaber, S. R. White, L. T. Peltenburg, K. F. Rabe, and P. S. Hiemstra. 2006. Mechanisms of cell death induced by the neutrophil antimicrobial peptides alpha-defensins and LL-37. Inflamm. Res. 55:119-127. [DOI] [PubMed] [Google Scholar]
- 2.Abraham, P. R., B. J. Appelmelk, and S. J. H. van Deventer. September 2003. Synthetic peptides with antimicrobial and endotoxin neutralizing properties for management of the sepsis syndrome. U.S. patent 6624140.
- 3.Balaban, N., Y. Gov, A. Giacometti, O. Cirioni, R. Ghiselli, F. Mocchegiani, F. Orlando, G. D'Amato, V. Saba, G. Scalise, S. Bernes, and A. Mor. 2004. A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci. Antimicrob. Agents Chemother. 48:2544-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals, R., X. Wang, M. Zasloff, and J. M. Wilson. 1998. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl. Acad. Sci. USA 95:9541-9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barie, P. S. 1998. Antibiotic-resistant gram-positive cocci: implications for surgical practice. World J. Surg. 22:118-126. [DOI] [PubMed] [Google Scholar]
- 6.Belas, R., J. Manos, and R. Suvanasuthi. 2004. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 72:5159-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boelens, J. J., J. Dankert, J. L. Murk, J. J. Weening, T. van der Poll, K. P. Dingemans, L. Koole, J. D. Laman, and S. A. J. Zaat. 2000. Biomaterial-associated persistence of Staphylococcus epidermidis in pericatheter macrophages. J. Infect. Dis. 181:1337-1349. [DOI] [PubMed] [Google Scholar]
- 8.Boelens, J. J., S. A. J. Zaat, J. Meeldijk, and J. Dankert. 2000. Subcutaneous abscess formation around catheters induced by viable and nonviable Staphylococcus epidermidis as well as by small amounts of bacterial cell wall components. J. Biomed. Mater. Res. 50:546-556. [DOI] [PubMed] [Google Scholar]
- 9.Boelens, J. J., S. A. J. Zaat, J. L. Murk, J. J. Weening, T. van der Poll, and J. Dankert. 2000. Enhanced susceptibility to subcutaneous abscess formation and persistent infection around catheters is associated with sustained interleukin-1beta levels. Infect. Immun. 68:1692-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boman, H. G., B. Agerberth, and A. Boman. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 61:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, G. D., W. A. Simpson, A. L. Bisno, and E. H. Beachey. 1982. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect. Immun. 37:318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cirioni, O., A. Giacometti, R. Ghiselli, G. Dell'Acqua, Y. Gov, W. Kamysz, J. Lukasiak, F. Mocchegiani, F. Orlando, G. D'Amato, N. Balaban, V. Saba, and G. Scalise. 2003. Prophylactic efficacy of topical temporin A and RNAIII-inhibiting peptide in a subcutaneous rat pouch model of graft infection attributable to staphylococci with intermediate resistance to glycopeptides. Circulation 108:767-771. [DOI] [PubMed] [Google Scholar]
- 13.Costerton, J. W., K. J. Cheng, G. G. Geesey, T. I. Ladd, J. C. Nickel, M. Dasgupta, and T. J. Marrie. 1987. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 41:435-464. [DOI] [PubMed] [Google Scholar]
- 14.Cox, A. D., and S. G. Wilkinson. 1991. Ionizing groups in lipopolysaccharides of Pseudomonas cepacia in relation to antibiotic resistance. Mol. Microbiol. 5:641-646. [DOI] [PubMed] [Google Scholar]
- 15.Da Costa, A., G. Kirkorian, M. Cucherat, F. Delahaye, P. Chevalier, A. Cerisier, K. Isaaz, and P. Touboul. 1998. Antibiotic prophylaxis for permanent pacemaker implantation: a meta-analysis. Circulation 97:1796-1801. [DOI] [PubMed] [Google Scholar]
- 16.Dankert, J., A. Hogt, and J. Feijen. 1986. Biomedical polymers: bacterial adhesion, colonization, and infection. CRC Crit. Rev. Biocompat. 2:219-301. [Google Scholar]
- 17.Dankert, J., J. Van der Werff, S. A. J. Zaat, W. Joldersma, D. Klein, and J. Hess. 1995. Involvement of bactericidal factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect. Immun. 63:663-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gotz, F., C. Heilmann, and S. E. Cramton. 2000. Molecular basis of catheter associated infections by staphylococci. Adv. Exp. Med. Biol. 485:103-111. [DOI] [PubMed] [Google Scholar]
- 19.Gristina, A. G., C. D. Hobgood, L. X. Webb, and Q. N. Myrvik. 1987. Adhesive colonization of biomaterials and antibiotic resistance. Biomaterials 8:423-426. [DOI] [PubMed] [Google Scholar]
- 20.Hancock, R. E. 2000. Cationic antimicrobial peptides: towards clinical applications. Expert Opin. Investig. Drugs 9:1723-1729. [DOI] [PubMed] [Google Scholar]
- 21.Hancock, R. E., and A. Patrzykat. 2002. Clinical development of cationic antimicrobial peptides: from natural to novel antibiotics. Curr. Drug Targets Infect. Disord. 2:79-83. [DOI] [PubMed] [Google Scholar]
- 22.Hancock, R. E., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:8856-8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanssen, A. D., and D. R. Osmon. 1999. The use of prophylactic antimicrobial agents during and after hip arthroplasty. Clin. Orthop. Relat. Res. 1999:124-138. [DOI] [PubMed] [Google Scholar]
- 24.Harwig, S. S., T. Ganz, and R. I. Lehrer. 1994. Neutrophil defensins: purification, characterization, and antimicrobial testing. Methods Enzymol. 236:160-172. [DOI] [PubMed] [Google Scholar]
- 25.Henke, P. K., T. M. Bergamini, S. M. Rose, and J. D. Richardson. 1998. Current options in prosthetic vascular graft infection. Am. Surg. 64:39-45. [PubMed] [Google Scholar]
- 26.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 27.Isaacson, R. E. 2003. MBI-226. Micrologix/Fujisawa. Curr. Opin. Investig. Drugs 4:999-1003. [PubMed] [Google Scholar]
- 28.Jenssen, H., P. Hamill, and R. E. Hancock. 2006. Peptide antimicrobial agents. Clin. Microbiol. Rev. 19:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi, S., K. Takeshima, C. B. Park, S. C. Kim, and K. Matsuzaki. 2000. Interactions of the novel antimicrobial peptide buforin 2 with lipid bilayers: proline as a translocation promoting factor. Biochemistry 39:8648-8654. [DOI] [PubMed] [Google Scholar]
- 30.Lehrer, R. I., A. Barton, K. A. Daher, S. S. Harwig, T. Ganz, and M. E. Selsted. 1989. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J. Clin. Investig. 84:553-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy, O. 2000. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 96:2664-2672. [PubMed] [Google Scholar]
- 32.Levy, S. B. 2002. Active efflux, a common mechanism for biocide and antibiotic resistance. J. Appl. Microbiol. 92(Suppl.):65S-71S. [PubMed] [Google Scholar]
- 33.Mack, D., N. Siemssen, and R. Laufs. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maisetta, G., G. Batoni, S. Esin, W. Florio, D. Bottai, F. Favilli, and M. Campa. 2006. In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob. Agents Chemother. 50:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Movahed, M. R., B. Kasravi, and C. S. Bryan. 2004. Prophylactic use of vancomycin in adult cardiology and cardiac surgery. J. Cardiovasc. Pharmacol. Ther. 9:13-20. [DOI] [PubMed] [Google Scholar]
- 36.Nagaoka, I., S. Hirota, S. Yomogida, A. Ohwada, and M. Hirata. 2000. Synergistic actions of antibacterial neutrophil defensins and cathelicidins. Inflamm. Res. 49:73-79. [DOI] [PubMed] [Google Scholar]
- 37.Sampath, L. A., S. M. Tambe, and S. M. Modak. 2001. In vitro and in vivo efficacy of catheters impregnated with antiseptics or antibiotics: evaluation of the risk of bacterial resistance to the antimicrobials in the catheters. Infect. Control Hosp. Epidemiol. 22:640-646. [DOI] [PubMed] [Google Scholar]
- 38.Schierholz, J. M., and J. Beuth. 2001. Implant infections: a haven for opportunistic bacteria. J. Hosp. Infect. 49:87-93. [DOI] [PubMed] [Google Scholar]
- 39.Seifert, H., B. Jansen, and B. Farr. 1997. Catheter-related infections. Marcel Dekker Press, New York, N.Y.
- 40.Shai, Y. 1999. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1462:55-70. [DOI] [PubMed] [Google Scholar]
- 41.Sidorczyk, Z., U. Zahringer, and E. T. Rietschel. 1983. Chemical structure of the lipid A component of the lipopolysaccharide from a Proteus mirabilis Re-mutant. Eur. J. Biochem. 137:15-22. [DOI] [PubMed] [Google Scholar]
- 42.Subbalakshmi, C., and N. Sitaram. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160:91-96. [DOI] [PubMed] [Google Scholar]
- 43.Tojo, M., N. Yamashita, D. A. Goldmann, and G. B. Pier. 1988. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J. Infect. Dis. 157:713-722. [DOI] [PubMed] [Google Scholar]
- 44.Tossi, A., L. Sandri, and A. Giangaspero. 2000. Amphipathic, alpha-helical antimicrobial peptides. Biopolymers 55:4-30. [DOI] [PubMed] [Google Scholar]
- 45.Turner, J., Y. Cho, N. N. Dinh, A. J. Waring, and R. I. Lehrer. 1998. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob. Agents Chemother. 42:2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Wetering, S., S. P. Mannesse-Lazeroms, M. A. van Sterkenburg, M. R. Daha, J. H. Dijkman, and P. S. Hiemstra. 1997. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 272:L888-L896. [DOI] [PubMed] [Google Scholar]
- 47.Vuong, C., S. Kocianova, Y. Yao, A. B. Carmody, and M. Otto. 2004. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J. Infect. Dis. 190:1498-1505. [DOI] [PubMed] [Google Scholar]
- 48.Waldvogel, F. A., and A. L. Bisno. 2000. Infections associated with indwelling medical devices. ASM Press, Washington, D.C.
- 49.Yang, L., T. M. Weiss, R. I. Lehrer, and H. W. Huang. 2000. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys. J. 79:2002-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, Q., R. I. Lehrer, and J. P. Tam. 2000. Engineered salt-insensitive alpha-defensins with end-to-end circularized structures. J. Biol. Chem. 275:3943-3949. [DOI] [PubMed] [Google Scholar]
- 51.Zaat, S. A. J., P. S. Hiemstra, and J. Dankert. 1994. Pathogenic streptococci, present and future, p. 473-475. Lancer Publication, St. Petersburg, Russia.
- 52.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, L., and T. J. Falla. 2004. Cationic antimicrobial peptides—an update. Expert Opin. Investig. Drugs 13:97-106. [DOI] [PubMed] [Google Scholar]