Abstract
The gut microbiota is critical for maturation of the immune system. Recent evidence suggests that early establishment of lactobacilli in the intestinal microbiota, during neonatal colonization or by probiotic supplementation, could prevent the development of allergic disorders. Postnatal maturation of the gut immune system with allergen-producing lactobacilli colonizing the digestive tract could then affect the development of further allergic sensitization. In this paper, we describe construction of a recombinant Lactobacillus casei strain that can constitutively deliver bovine β-lactoglobulin (BLG), a major cow's milk allergen, to the guts of gnotobiotic mice. The blg gene was inserted into the L. casei chromosome downstream of an endogenous promoter. BLG production was improved by fusing the propeptide LEISSTCDA (LEISS) to the BLG mature moiety. This led to a 10-fold increase in LEISS-BLG production compared to the production obtained without the propeptide and also led to enhanced secretion corresponding to 5% of the total production. After inoculation into germfree C3H/HeN mice, the genetic stability of the recombinant strain and in vivo BLG production were confirmed for at least 10 weeks. BLG stimulation of spleen cells from mice monoassociated with the BLG-producing lactobacilli induced secretion of the Th1 cytokine gamma interferon and, to a lesser extent, the Th2 cytokine interleukin-5. No BLG-specific immunoglobulin G1 (IgG1), IgG2a, or IgA was detected in sera or in fecal samples. These results suggest that gut colonization with allergen-producing lactobacilli could provide a useful model for studying the modulation of allergic disorders.
Food allergy affects 1 to 2% of adults and 5 to 8% of children in Western countries (25). With an incidence of 1.9 to 2.8%, cow's milk allergy is the most common allergy in early infancy (13). Patients may be sensitized to various proteins, mainly β-lactoglobulin (BLG) and caseins (39). Food allergy generally corresponds to an inappropriate immune response characterized by disruption of the Th1/Th2 balance toward a Th2 profile that results in the production of immunoglobulins E (IgE) specific for food antigens. Th2 cells produce interleukin-4 (IL-4), IL-5, and IL-13, whereas the Th1 response is characterized by gamma interferon (IFN-γ) and IL-12 synthesis. The Th1 and Th2 responses inhibit each other's development and function via the cytokines produced (26).
Development of allergy is multifactorial, and it includes genetic factors and also different environmental factors, such as lifestyle and the intestinal microbiota. The intestinal microbiota seems to be critical because of its role in the postnatal maturation of the immune system. At birth, the digestive tract is sterile and the neonatal immune response is characterized by a polarized Th2 cytokine profile. During postnatal gut colonization, the gut immune system is exposed to a wide range of bacterial antigens, which apparently play a major role in driving the initial Th2-skewed immune response toward a more finely balanced Th1/Th2 response (5). Oral tolerance to BLG or ovalbumin can also be promoted by monocolonization of the guts of germfree rodents with Bifidobacterium infantis, Lactobacillus paracasei, or Escherichia coli but not by monocolonization with Clostridium perfringens or Staphylococcus aureus, thus demonstrating that there is a species- and strain-dependent effect (34, 38). Smaller amounts of lactobacilli and bifidobacteria have been found in the intestinal microbiota of allergic children than in the intestinal microbiota of nonallergic children (5). Clinical studies have also shown that administration of lactobacilli can alleviate the intestinal inflammation caused by food allergy (18, 35).
In addition to their intrinsic adjuvanticity, lactobacilli, and more generally lactic acid bacteria (LAB), are also attractive vectors for delivery of therapeutic proteins to the digestive tract. In our laboratory, recombinant Lactococcus lactis strains have been developed for production of bovine BLG, a major cow's milk allergen, by use of the nisin-inducible expression system (6, 7). Oral administration of these recombinant L. lactis strains to conventional mice has been shown to promote a Th1 response down-regulating a further Th2 response induced by intraperitoneal injection of BLG (2). When purified BLG was administered with a control L. lactis strain, oral tolerance was abrogated, further demonstrating the adjuvant role of this LAB (2). These results show the potential of recombinant LAB for modulation of food allergies. However, because of the resident gut microbiota, the presence of ingested LAB is transient. Moreover, uptake of a pure L. lactis culture leads to massive lysis of the strain in each compartment of the digestive tract (10). Here, we wanted to investigate the effect of an allergen-producing LAB established permanently in the guts of gnotobiotic mice. Because of its substantial survival rate and its high metabolic activity in the digestive environment (31), Lactobacillus casei appears to be a good candidate for gut colonization and for delivery of therapeutic proteins to the gut mucosal system.
In the present work, we engineered a strain of L. casei that could deliver BLG continuously to the digestive tract. For this purpose, we fused the blg gene to a partial lacTEGF operon promoter. The lacTEGF operon encodes an antiterminator protein (LacT), lactose-specific phosphoenolpyruvate-dependent phosphotransferase system proteins (LacE and LacF), and a phospho-β-galactosidase (LacG) (4, 15, 32). The recombinant L. casei strain was subsequently administered orally to germfree C3H/HeN mice, and production of BLG in the digestive tracts of these mice was monitored for 10 weeks. Furthermore, we determined whether gut colonization with the recombinant L. casei strain could stimulate an immune response against BLG.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Escherichia coli strain TG1-RepA was used for plasmid propagation (14). It was cultured aerobically in Luria-Bertani broth at 37°C. L. casei strains, derived from strain BL23 (ATCC 393 cured of plasmid pLZ15 [1]), were cultured at 37°C in De Man-Rogosa-Sharpe (MRS) broth (9) (Difco, BD, Le Pont de Claix, France). Plates were incubated in anaerobic jars for 2 days at 37°C in an Anaerocult A system (Merck). When required, erythromycin (Merck, Darmstadt, Germany) was added to the media at concentrations of 100 μg/ml and 5 μg/ml to select E. coli and L. casei transformants, respectively.
DNA manipulations.
Purification of genomic DNA from L. casei was performed using a NucleoSpin tissue kit (Macherey-Nagel, Hoerdt, France). Restriction enzymes and T4 DNA ligase were obtained from Fermentas (Vilnius, Lithuania), and Taq DNA polymerase was purchased from TakaraBio, Inc. (Otsu Shiga, Japan). L. casei was transformed by electroporation using a gene pulser apparatus (Bio-Rad Laboratories, Richmond, Calif.) as previously described (33).
Plasmids and strain constructs.
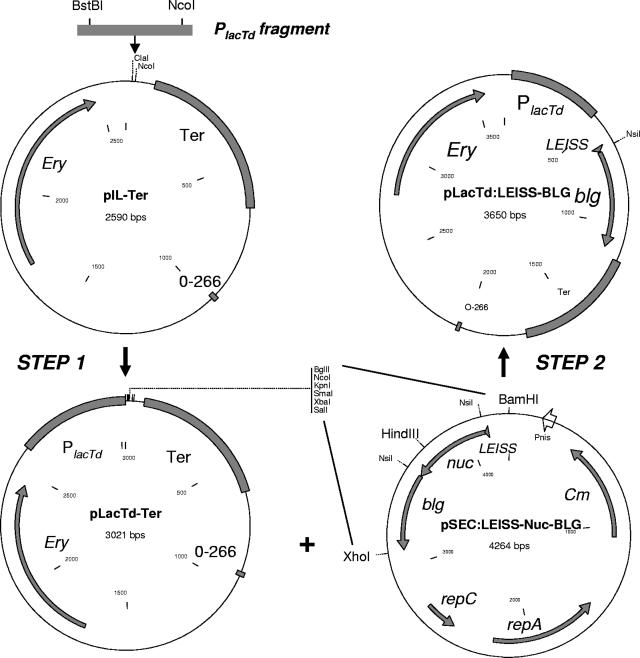
Plasmid pIL4242 was kindly provided by Jamila Anba (30). Plasmid pIL-Ter was obtained by removing the P45 promoter and luxAB genes by XmaI cleavage and self-ligation (Table 1). A transcriptional fusion of the L. casei lacTEGF operon promoter with a cassette for constitutive synthesis of bovine BLG was constructed (Fig. 1). The sequence of the lacTEGF operon from strain BL23, available from GenBank (accession no. Z80834), was used to design primers LacTdFOR (5′-TGTTCGAAGTAGAGGCATCGTCGATGAGCC-3′) and LacTdREV (5′-GATCCATGGAGATCTCGCCTAATTAAATAGTCAC-3′). The 452-bp PCR fragment obtained with the two primers contained the lac operon promoter (PlacTd) without the stem-loop structure involved in lactose-dependent induction. The PCR fragment was digested with NcoI and BstBI and fused to pIL-Ter cut by NcoI and ClaI. The resulting plasmid, pLacTd-Ter, cut by BglII and SalI, was then fused to BamHI-HindIII and HindIII-XhoI fragments from pSEC:Nuc-BLG or pSEC:LEISS-Nuc-BLG (28). The sequence encoding the Nuc protein was removed by NsiI digestion, resulting in plasmids pLacTd:BLG and pLacTd:LEISS-BLG. These plasmids contained a transcriptional fusion of the PlacTd promoter with the production cassette for BLG fused or not fused to the synthetic propeptide LEISSTCDA (LEISS)(20). Control plasmid pLacTd:Cont was obtained by fusing pLacTd-Ter cut by BglII with the BamHI fragment of pNZ9520, which contained the nisRK genes (19).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Characteristics | Reference |
|---|---|---|
| Strains | ||
| E. coli TG1 RepA | TG1 with repA from E. coli EC101 integrated into the chromosome | 14 |
| L. casei | ||
| BL23 | L. casei ATCC 393(pLZ15−) | 1 |
| BLacTd:BLG | BL23, Emr, with pLacTd:BLG integrated into the chromosome | This study |
| BLacTd:LEISS-BLG | BL23, Emr, with pLacTd:LEISS-BLG integrated into the chromosome | This study |
| BLacTd:Cont | BL23, Emr, with pLacTd:Cont integrated into the chromosome | This study |
| Plasmids | ||
| pIL4242 | Emr, ori(pWV01 ΔrepA), integrative vector | 29 |
| pIL-Ter | Emr, ori(pWV01 ΔrepA), pIL4242 with the P45 promoter and luxAB genes deleted | This study |
| pLacTd-Ter | Emr, ori(pWV01 ΔrepA), derivative of pIL-Ter containing the 452-bp fragment encoding PlacTd without the ribonucleic antiterminator sequences | This study |
| pSEC:Nuc-BLG | Cmr, ori(pWV01), with a DNA fragment encoding the precursor SPUsp45-Nuc-BLG expressed under transcriptional control of the nisin-inducible promoter PnisA | 28 |
| pSEC:LEISS-Nuc-BLG | Cmr, ori(pWV01), with a DNA fragment encoding the precursor SPUsp45-LEISS-Nuc-BLG expressed under transcriptional control of the nisin-inducible promoter PnisA | 28 |
| pLacTd:BLG | Emr, ori(pWV01 ΔrepA), derivative of pLacTd-Ter with BLG cassette expression | This study |
| pLacTd:LEISS-BLG | Emr, ori(pWV01 ΔrepA), derivative of pLacTd-Ter with LEISS-BLG cassette expression | This study |
| pLacTd:Cont | Emr, ori(pWV01 ΔrepA), derivative of pLacTd-Ter with nisRK genes | This study |
| pNZ9520 | Emr, nisRK cloned in pIL253 | 19 |
| pVE6007 | Cmr, thermosensitive derivative of pGKV12 | 24 |
FIG. 1.
Genetic constructs. Step 1 is PlacTd promoter insertion. After PCR amplification, the PlacTd fragment, cut by BstBI-NcoI, was fused to plasmid pIL-Ter cut by NcoI-ClaI. Step 2 is BLG expression cassette insertion. pLacTd-Ter, cut by BglII-SalI, was fused to the BamHI-HindIII and HindIII-XhoI fragments from pSEC:LEISS-Nuc-BLG. The Nuc-encoding sequence was then deleted by NsiI digestion, resulting in plasmid pLacTd:LEISS-BLG (see Materials and Methods).
The pLacTd:BLG and pLacTd:LEISS-BLG plasmids were used to integrate the construct into the L. casei chromosome with a recombination event occurring at the PlacTd locus. L. casei was first transformed with pVE6007, a thermosensitive plasmid used as a helper plasmid to provide the RepA replicase for plasmid replication (24). The strain was then transformed with pLacTd:BLG, pLacTd:LEISS-BLG, or pLacTd:Cont, which could replicate in the presence of pVE6007 at the permissive temperature (30°C). A shift to the restrictive temperature (42°C) inhibited plasmid pVE6007 replication and allowed us to select integrants which acquired erythromycin resistance because of blg-encoding plasmid insertion into the chromosome by homologous recombination. Integrants were analyzed by PCR with primers OFF43 (5′-GGTCATTAAAACCGGTCGCGTG-3′), located upstream of PlacT, and OFF43r (5′-TTCCCAGTCACGACGTTG-3′), located downstream of the blg gene, to check for correct insertion into the chromosome.
Cell extracts, Western blot analysis, and immunoassay for BLG.
An overnight culture of the L. casei strain was used to inoculate fresh medium at a dilution of 1:20. Strains were grown until the optical density at 600 nm was 2. L. casei cultures (2 ml) were pelleted by centrifugation at 8,000 × g for 5 min at 4°C. Each pellet was washed once and resuspended in 100 μl of 50 mM Tris-HCl (pH 7.5)-5 mM EDTA. Cells were disrupted with glass beads, and 100 μl of 2× Laemmli buffer (supplemented with 5% β-mercaptoethanol as a reducing agent) was added. Samples were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis followed by Western blotting using specific anti-BLG monoclonal antibody Blg-92R (27).
For the immunoassay, cells were pelleted by centrifugation at 8,000 × g for 5 min at 4°C, and the supernatant (S fraction) was collected. Cells were resuspended in 50 mM Tris-HCl (pH 7.5)-5 mM EDTA, and the soluble cytoplasmic protein (Cs fraction) was extracted by disrupting cells with glass beads. After centrifugation (15,000 × g, 15 min, 4°C), the supernatant corresponding to the Cs fraction was collected. The pellet was resuspended in 50 mM Tris-HCl (pH 7.5)-8 M urea-100 mM dithiothreitol and incubated for 1 h at room temperature. After centrifugation (15,000 × g, 15 min, 4°C), the supernatant containing the resolubilized insoluble cytoplasmic protein (Ci fraction) was collected and dialyzed against 20 mM Tris-HCl (pH 7.5) by using a floating filter disk with a pore size of 0.025 μm (Millipore Corporation, Bedford, MA). The amounts of the BLG native (BLGn) and denatured (BLGd) forms in the S, Cs and Ci fractions were determined as previously described (27). Briefly, 96-well microtiter plates (Maxisorp; Nunc, Roskilde, Denmark) were coated with a first monoclonal antibody (capture antibody) specific for BLGn or BLGd. Then 50 μl of a sample and 50 μl of a tracer (second monoclonal antibody labeled with acetylcholinesterase [AChE]) were added. Native BLG and reduced and carboxymethylated BLG were used as standards for quantification of BLGn and BLGd, respectively. After 18 h of incubation at 4°C, the plates were extensively washed, and solid-phase-bound AChE activity was measured by the method of Ellman et al. (11). The intracellular BLG concentration was also calculated with respect to the total protein determined by the bicinchoninic acid protein assay (Pierce).
Protocol for animal experiments.
Eighteen female adult C3H/HeN mice were used. They were born germfree and bred in germfree conditions in our breeding facilities, using established methods (8). Animals were 8 weeks old at the start of the experiment. In order to prevent contamination of the gut microbiota by external bacteria, the mice were housed in Trexler-type isolators (La Calhène, Vélizy, France). Autoclaved tap water and pelleted standard chow (R03; SAFE, Augy, France) sterilized by γ irradiation at 45 kGy (IBA Mediris, Fleurus, Belgium) were given ad libitum. The room housing the isolators was maintained at a constant temperature (21 ± 1°C) and constant humidity (50% ± 5%) with a cycle consisting of 12 h of light and 12 h of darkness.
Overnight cultures of recombinant strains were used to inoculate fresh MRS broth at a dilution of 1:10. After incubation for 2 h at 37°C, 0.2 ml of a fresh culture containing approximately 108 CFU was administered to germfree mice by intragastric intubation. Two groups of six germfree mice were inoculated on days 1 and 6 with the BLacTd:Cont and BLacTd:LEISS-BLG strains, respectively. Colonization was monitored by fecal bacterial counting performed on nonselective MRS medium and on MRS medium supplemented with 5 μg/ml erythromycin. Bacterial counts were evaluated once a week for the first 3 weeks and then every 2 weeks until the end of the experiment. In parallel, a group of six germfree mice was kept as a control in a sterile environment. Serum and fecal samples were collected for antibody analysis on days 28, 47, and 63. On day 68, mice were killed, and spleens were collected for cytokine analysis. All experiments were performed in accordance with European Community rules for animal care and with permission 91-244 of the French Veterinary Services.
Isolation of bacteria from feces.
In parallel with the fecal bacterial counting, fresh feces from the BLacTd:LEISS-BLG and BLacTd:Cont mouse groups were pooled (3 g for each group) and stored at −80°C. These samples were used to assess in vivo BLG synthesis by the BLacTd:LEISS-BLG strain. Feces were suspended in 10 ml of sterile phosphate-buffered saline (PBS) and homogenized with an Ultra-turrax (Bioblock, Paris, France). The suspension was centrifuged at low speed (200 × g, 5 min, 4°C) in order to remove fecal debris. The supernatant was collected and centrifuged at 5,500 × g for 20 min at 4°C. The pellet containing the bacterial fraction was then resuspended in 4 ml of PBS. L. casei cells were isolated from feces particles using high-speed centrifugation on a Nycodenz density gradient (Axis-shield PoC; AS, Oslo, Norway). Four milliliters of 50% (wt/vol) Nycodenz in PBS was gently placed at the bottom of the fecal suspension in a centrifuge tube, and this was followed by centrifugation at 10,000 × g in Beckman SWTi-40 swing-out rotor for 1 h at 4°C. The layer of packed cells at the interface was carefully recovered. The Nycodenz solution was removed from the bacteria by diluting the suspension in sterile PBS before centrifugation at 5,500 × g for 20 min at 4°C. The cell pellet was washed twice and resuspended in 50 mM Tris-HCl (pH 7.5)-5 mM EDTA. Protein extraction from bacterial cells, Western blot analysis (with a rabbit polyclonal serum against BLG as the primary antibody), and immunometric assays for BLG were performed as described above.
Quantification of BLG-specific serum antibodies.
BLG-specific IgG1, IgG2a, and IgA were measured as previously described (3). Briefly, 96-well microtiter plates coated with 5 μg/ml BLG were incubated overnight with serial dilutions of sera in EIA buffer (0.1 M phosphate buffer, 0.1% bovine serum albumin, 0.15 M NaCl, 0.01% sodium azide). After washing, IgG1, IgG2a, and IgA binding was revealed by incubation with goat anti-mouse IgG1, IgG2a, or IgA (Southern Biotechnology Associates, Birmingham, AL) labeled with AChE (16).
Quantification of BLG-specific sIgA in fecal extracts.
Fresh fecal pellets were collected from mice, added to PBS containing 50 μg/ml bacitracin, 300 μg/ml benzamidin, 80 μg/ml leupeptin, 20 μg/ml chymostatin, 25 μg/ml pepstatin, and 200 μM phenylmethylsulfonyl fluoride (Sigma), and incubated on a rotary shaker overnight at 4°C. Suspensions were then centrifuged at 15,000 × g for 10 min at 4°C. Each supernatant was collected, and the total protein concentration was determined by the bicinchoninic acid protein assay (Pierce). Fecal samples (0.1 mg protein/ml), prepared in EIA buffer containing 0.1% Tween, were incubated on plates coated with 5 μg/ml BLG. Specific secretory IgA (sIgA) was detected using a goat polyclonal anti-mouse IgA serum (Southern Biotechnology Associates, Birmingham, AL) labeled with AChE as described above.
Cytokine production.
On day 68, spleens were harvested under sterile conditions and pooled in RPMI-10 (RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin). After lysis of red blood cells (180 mM NH4Cl, 17 mM Na2EDTA) and several washes, splenocytes were resuspended in RPMI-10. Cells were incubated for 60 h at 37°C in the presence of 5% CO2 in 96-well culture plates (in quadruplicate with 106 cells/well) in the presence of BLG (20 μg/ml), concanavalin A (ConA) (1 μg/ml; positive control), and ovalbumin (20 μg/ml; negative control). After incubation, culture plates were centrifuged, and supernatants were collected and stored at −80°C until they were assayed.
IL-4 and IFN-γ were assayed using CytoSets kits (Biosource International Europe, Nivelles, Belgium). IL-5 was assayed as previously described, using TRFK4 monoclonal antibodies for capture and AChE-labeled TRFK5 monoclonal antibodies for development (12).
RESULTS
BLG production in L. casei.
To obtain constitutive synthesis of BLG in L. casei, we used a transcriptional fusion between the blg gene and the lacTEGF operon promoter with the ribonucleic antiterminator sequences deleted (see Materials and Methods). Two integrative plasmids, pLacTd:BLG and pLacTd:LEISS-BLG, were generated (Fig. 1). These constructs contained expression cassettes for production of bovine BLG or a fusion protein between BLG and the LEISSTCDA propeptide (Fig. 2A). The precursor forms of the recombinant proteins also contained the signal peptide of the major L. lactis secreted protein, Usp45, in order to direct secretion of BLG (6). Plasmids were then integrated into the L. casei chromosome by single-crossover homologous recombination (see Materials and Methods). The corresponding strains are listed in Table 1.
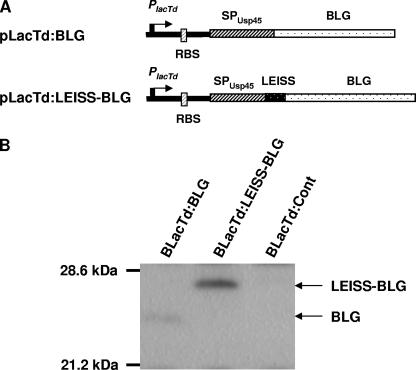
FIG. 2.
(A) Expression cassettes for production and secretion of BLG. PlacTd, partial lac promoter; RBS, ribosome binding site; SPUsp45, signal peptide of Usp45; LEISS, secretion enhancer propeptide LEISSTCDA; BLG, sequence encoding the mature bovine β-lactoglobulin. (B) Western blot analysis of L. casei strains BLacTd:BLG and BLacTd:LEISS-BLG. Protein extracts were analyzed by immunoblotting using a specific anti-BLG monoclonal antibody as the primary antibody.
Total protein extracts were prepared from the recombinant strains and were analyzed by Western blotting with anti-BLG monoclonal antibodies (Fig. 2B). A specific band corresponding to the precursor form was observed at around 25 kDa for the BLG-producing strain, while a more intense band at a higher molecular weight was observed for the LEISS-BLG-producing strain. No specific band was detected with the BLacTd:Cont strain. BLG production was also assayed using immunoassays specific for the native and denatured forms of BLG in the S, Cs, and Ci fractions (Table 2). The level of BLG production by BLacTd:BLG was estimated to be about 0.2 μg/liter of culture. BLG was found exclusively in a denatured form and in the Cs fraction. Fusion of the LEISSTCDA propeptide to BLG led to a 10-fold increase in total BLG production, which reached 2.2 μg/liter of culture. Around 87% of the BLG synthesized by BLacTd:LEISS-BLG was detected in the soluble cytoplasmic fraction, and most of it (97.5%) was in a denatured form. The intracellular BLG concentration was calculated with respect to the total protein and corresponded to around 10.5 ng of BLG per mg of total protein. In addition to enhanced synthesis, the presence of the LEISS propeptide allowed secretion of BLG, only in its native form, at a level corresponding to 5% of the total BLG production. Considering the higher level of BLG production and secretion, the BLacTd:LEISS-BLG strain appeared to be a better candidate than the BLacTd:BLG strain for delivery of BLG to mucosal sites. Therefore, the experiments described below were performed with the BLacTd:LEISS-BLG strain.
TABLE 2.
Quantitative assay of native and denatured BLG in different fractions of recombinant L. casei strains
| Fraction | BLG | Concn (μg/liter of culture)a
|
||
|---|---|---|---|---|
| BlacTd:BLG | BLacTd:LEISS-BLG | BLacTd:Cont | ||
| S | BLGn | NDb | 0.11 ± 0.01 | ND |
| BLGd | ND | ND | ND | |
| Cs | BLGn | ND | 0.05 ± 0.03 | ND |
| BLGd | 0.21 ± 0.04 | 1.87 ± 1.10 | ND | |
| Ci | BLGd | ND | 0.17 ± 0.11 | ND |
| Total | BLGn + BLGd | 0.21 ± 0.04 | 2.19 ± 1.04 | ND |
The values are the means ± standard deviations for two (BLacTd:BLG) or four (BLacTd:LEISS-BLG) independent experiments.
ND, not detectable.
Gut colonization of germfree mice by the BLacTd:LEISS-BLG strain.
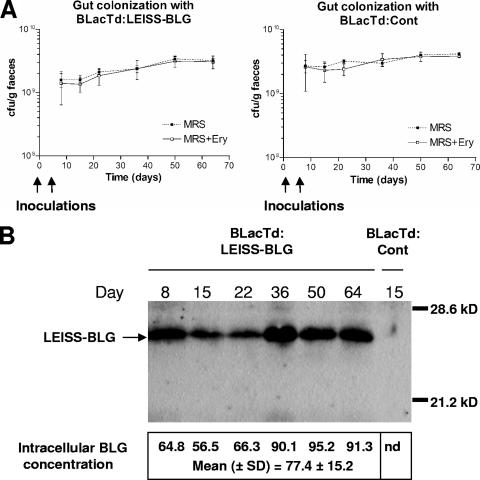
After intragastric administration of the recombinant strains on day 1 and 6, the level of colonization of the gut by L. casei was evaluated by bacterial fecal counting. The BLacTd:LEISS-BLG and BLacTd:Cont strains were able to colonize the guts of mice at similar levels (Fig. 3A). A week after inoculation, the level of gut colonization was more than 109 CFU/g of feces and remained stable thereafter at around 3 × 109 CFU/g feces. Throughout the experiment, both strains were found to be resistant to erythromycin, indicating that they still possessed the integrated plasmid.
FIG. 3.
(A) Bacterial counts in MRS medium with or without erythromycin (Ery) (5 μg/ml) for fecal pellets from mice monoassociated with the BLacTd:Cont or the BLacTd:LEISS-BLG strain. The data are expressed as means ± standard deviations for six mice per time. (B) Western blot analysis of protein extracts from bacteria isolated from pooled feces (see Materials and Methods). The intracellular BLG concentration is expressed in ng of BLG per mg of total protein. nd, not detectable.
In parallel, we estimated BLG production in bacteria isolated from feces in order to verify the ability of the BLacTd:LEISS-BLG strain to produce BLG in vivo. Bacterial cells were recovered from feces, and total protein extracts were characterized by immunoblot analysis (Fig. 3B). BLG synthesis in bacteria established in the guts of mice monoassociated with BLacTd:LEISS-BLG was confirmed throughout the experiment. The recombinant BLG was detected only in its denatured form, as determined by immunoassays (data not shown). Around 70% of the total BLG production was found in the Cs fraction, and the intracellular BLG concentration was estimated to be 77 ng of BLG per mg of total protein, which is sevenfold higher than the concentration found in bacteria grown in MRS broth (Fig. 3B).
Antibody response.
BLG-specific IgG1 and IgG2a were assayed in sera, while BLG-specific IgA was assayed in sera and fecal samples. No specific immunoglobulin response was detected for any group of mice, whether they were germfree or monocolonized with a recombinant L. casei.
Cytokines secreted after in vitro reactivation of splenocytes.
Germfree mice and gnotobiotic mice belonging to the BLacTd:Cont-treated group and the BLacTd:LEISS-BLG-treated group were killed at day 68, and spleens were pooled. In vitro cytokine production after ConA and BLG stimulation of spleen cells is shown in Fig. 4.
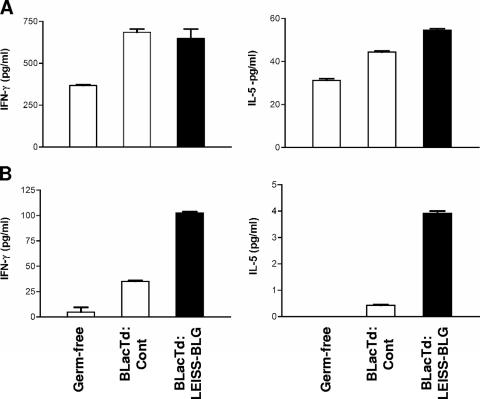
FIG. 4.
Specific IFN-γ and IL-5 cytokine secretion by concanavalin A-reactivated (A) and BLG-reactivated (B) splenocytes from germfree mice and from mice monoassociated with the BLacTd:Cont or BLacTd:LEISS-BLG strain. At the end of the experiment, mice were killed and spleens were removed and pooled (n = 6) for splenocyte reactivation (see Materials and Methods). IFN-γ and IL-5 secretions are expressed as cytokine secretion in supernatants of concanavalin A- or BLG-reactivated splenocytes after subtraction of data for cytokines assayed in supernatants of ovalbumin-reactivated splenocytes, thus corresponding to specific production. The values are means ± standard deviations for duplicate determinations.
We observed that monoassociation with the L. casei strains activated the host's cellular immune system. Indeed, stimulation by polyclonal ConA resulted in stronger secretion of IFN-γ and IL-5 in supernatants of spleen cell cultures from mice monoassociated with L. casei than in supernatants from germfree mice (Fig. 4A). However, whereas ConA induced similar levels of cytokine release, the IFN-γ secretion after BLG stimulation of splenocytes from BLacTd:LEISS-BLG-treated mice was markedly enhanced compared to the IFN-γ secretion observed for splenocytes from BLacTd:Cont-treated mice (Fig. 4B). Small amounts of IL-5 were also detected (Fig. 4B). No differences in IL-4 secretion were observed between the different groups of mice (data not shown).
DISCUSSION
It is thought that a major problem with antigen delivery by recombinant lactobacilli is the lower levels of antigen production compared to the levels obtained with other LAB, such as L. lactis (36). Taking advantage of our experience with BLG production (6, 28), we tested in L. casei several constructs that have been shown to enhance BLG synthesis in L. lactis. The improvements consisted of fusing BLG to the signal peptide of the major lactococcal secreted protein Usp45 in order to direct BLG to the cell membrane and insertion of the propeptide LEISSTCDA between SPUsp45 and BLG to enhance secretion. We found that the presence of LEISSTCDA led to a 10-fold increase in the production of the fusion protein. This effect was attributed in L. lactis to the presence of negatively charged residues in the N terminus of the mature moiety (21). Secretion was also enhanced since around 5% of the total BLG was secreted into the culture medium, which is similar to the secretion efficiency obtained for L. lactis (28). Thus, the production and secretion system initially developed with L. lactis is also functional in an L. casei strain. The improvement obtained in the present study could be of great interest for future applications with recombinant lactobacilli since we previously observed a direct correlation between the amounts of BLG produced by L. lactis and the modulation of immune responses in our mouse model of allergy (2). Here we must take into consideration the fact that the BLacTd:LEISS-BLG strain is designed for gut colonization of germfree mice. Consequently, BLG production is certainly limited by the use of a constitutive expression system and the presence of only one copy of the blg gene on the L. casei chromosome. Other applications with conventional mice, whose resident microbiota prevents survival of ingested LAB in the gut, could require a larger amount of antigen. In this case, the use of the nisin-inducible expression system on a multicopy plasmid (28), in combination with the improvements described above, should result in higher yields of recombinant protein in L. casei.
The recombinant L. casei strains were shown to successfully colonize the mouse gut and to be genetically stable for at least 10 weeks. Moreover, we confirmed that the BLacTd:LEISS-BLG strain was able to efficiently synthesize BLG in the digestive tract. In fact, this environment appeared to be particularly favorable for BLG synthesis since we observed a sevenfold increase in the intracellular BLG concentration in bacteria isolated from feces compared to the concentration in bacteria grown in MRS broth. This is in agreement with results of Oozeer et al. (29) showing that the level of transcription of a reporter gene whose expression is under control of the PlacTd promoter is high during intestinal transit (29). In comparison, a recombinant strain of L. lactis has been shown to produce recombinant mouse IL-10 in the colon at a level lower than the level observed in culture (37). We thus confirmed that the physiology of the BL23 strain is well adapted to the digestive tract environment and is particularly suited for delivery of proteins of interest in vivo. Colonization of the guts of germfree mice by the BLG-producing strain should therefore result in constant exposure of the gut immune system to BLG. In fact, we also expect that continuous delivery of BLG is achieved not only by BLG secretion but also by L. casei lysis in the lumen. Moreover, during the first weeks of colonization of the guts of germfree mice, bacterial translocation is very intense, as previously described (17), and thereby should enhance exposure of major components of the gut immune system, such as Peyer's patches, dendritic cells, and mesenteric lymph nodes, to BLG.
No antibody response against BLG was detected in sera or fecal samples from any group of mice at any time. We cannot eliminate the possibility that some BLG-specific fecal sIgA remained complexed with BLG produced in vivo or at the surface of the L. casei recombinant and thus was not available for immunoassay. The limited level of BLG production in the BLacTd:LEISS-BLG strain could also explain the absence of systemic and local antibody responses. On the other hand, we observed enhanced secretion of IFN-γ and IL-5 after in vitro BLG stimulation of spleen cells from mice monoassociated with BLacTd:LEISS-BLG compared to the secretion by splenocytes from mice monoassociated with BLacTd:Cont. The marked secretion of IFN-γ suggests that BLacTd:LEISS-BLG has some Th1-promoting properties. These data are consistent with a preliminary evaluation of the adjuvant effect of the BL23 strain. In vitro stimulation of naïve spleen cells from conventional mice with L. casei protein leads to substantial production of IFN-γ (data not shown). However, enhanced secretion of IL-5 also suggests that gut colonization by BLacTd:LEISS-BLG did not induce a strongly polarized response of the immune system but rather induced a mixed Th1/Th2 response. Taken together, the absence of systemic antibody responses could also reflect the fact that our recombinant L. casei strain acts as a natural gut commensal. In conventional mice, some Lactobacillus strains have been shown to have little effect on the systemic humoral response, possibly because of their similarities to gut commensals and their low immunogenicity compared to that of pathogenic bacteria (22, 23). In our model, the induction of a mixed Th1/Th2 cellular response without production of systemic antibodies could be the result of a well-balanced immune response against BLG that may counteract further pathological response.
In summary, our results suggest that colonization of the guts of germfree mice by the BLG-producing lactobacilli could influence the maturation of the gut immune system and specifically affect the development of an immune response against BLG. However, the effectiveness in alleviating clinical symptoms of allergic disease still needs to be investigated. Studies are currently being conducted with germfree and gnotobiotic mice in order to determine if gut colonization by a BLG-producing L. casei strain could modulate the allergic reaction to BLG.
Acknowledgments
We thank Anne Foussier and Pascal Guillaume for careful breeding of germfree mice and Marie-Claude Muller, Aurélia Bruneau, and Sandrine Ah Leung for skilled technical assistance.
Footnotes
Published ahead of print on 22 September 2006.
REFERENCES
- 1.Acedo-Félix, E., and G. Perez-Martinez. 2003. Significant differences between Lactobacillus casei subsp. casei ATCC 393T and a commonly used plasmid-cured derivative revealed by a polyphasic study. Int. J. Syst. Evol. Microbiol. 53:67-75. [DOI] [PubMed] [Google Scholar]
- 2.Adel-Patient, K., S. Ah-Leung, C. Creminon, S. Nouaille, J. M. Chatel, P. Langella, and J. M. Wal. 2005. Oral administration of recombinant Lactococcus lactis expressing bovine beta-lactoglobulin partially prevents mice from sensitization. Clin. Exp. Allergy 35:539-546. [DOI] [PubMed] [Google Scholar]
- 3.Adel-Patient, K., C. Creminon, H. Bernard, G. Clement, L. Negroni, Y. Frobert, J. Grassi, J. M. Wal, and J. M. Chatel. 2000. Evaluation of a high IgE-responder mouse model of allergy to bovine beta-lactoglobulin (BLG): development of sandwich immunoassays for total and allergen-specific IgE, IgG1 and IgG2a in BLG-sensitized mice. J. Immunol. Methods 235:21-32. [DOI] [PubMed] [Google Scholar]
- 4.Alpert, C. A., and U. Siebers. 1997. The lac operon of Lactobacillus casei contains lacT, a gene coding for a protein of the Bg1G family of transcriptional antiterminators. J. Bacteriol. 179:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Björkstén, B. 1999. The intrauterine and postnatal environments. J. Allergy Clin. Immunol. 104:1119-1127. [DOI] [PubMed] [Google Scholar]
- 6.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatel, J. M., S. Nouaille, K. Adel-Patient, Y. Le Loir, H. Boe, A. Gruss, J. M. Wal, and P. Langella. 2003. Characterization of a Lactococcus lactis strain that secretes a major epitope of bovine beta-lactoglobulin and evaluation of its immunogenicity in mice. Appl. Environ. Microbiol. 69:6620-6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates, M. E. 1968. The germ-free animal in research. Academic Press, London, United Kingdom.
- 9.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 10.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellman, G. L., K. D. Courtney, V. Andres, Jr., and R. M. Featherstone. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 7:88-95. [DOI] [PubMed] [Google Scholar]
- 12.Eum, S. Y., S. Haile, J. Lefort, M. Huerre, and B. B. Vargaftig. 1995. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin 5-dependent bronchial hyperresponsiveness. Proc. Natl. Acad. Sci. USA 92:12290-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Commission. 1998. Scientific co-operation on questions relating to food: consideration of the epidemiological basis for appropriate measures for the protection of public health in respect to food allergy. European Commission report T72. European Commission, Brussels, Belgium.
- 14.Gilson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 15.Gosalbes, M. J., V. Monedero, C. A. Alpert, and G. Perez-Martinez. 1997. Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol. Lett. 148:83-89. [DOI] [PubMed] [Google Scholar]
- 16.Grassi, J., Y. Frobert, P. Pradelles, F. Chercuitte, D. Gruaz, J. M. Dayer, and P. E. Poubelle. 1989. Production of monoclonal antibodies against interleukin-1 alpha and -1 beta. Development of two enzyme immunometric assays (EIA) using acetylcholinesterase and their application to biological media. J. Immunol. Methods 123:193-210. [DOI] [PubMed] [Google Scholar]
- 17.Ibnou-Zekri, N., S. Blum, E. J. Schiffrin, and T. von der Weid. 2003. Divergent patterns of colonization and immune response elicited from two intestinal Lactobacillus strains that display similar properties in vitro. Infect. Immun. 71:428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isolauri, E., T. Arvola, Y. Sutas, E. Moilanen, and S. Salminen. 2000. Probiotics in the management of atopic eczema. Clin. Exp. Allergy 30:1604-1610. [DOI] [PubMed] [Google Scholar]
- 19.Kleerebezem, M., M. M. Beerthuyzen, E. E. Vaughan, W. M. de Vos, and O. P. Kuipers. 1997. Controlled gene expression systems for lactic acid bacteria: transferable nisin-inducible expression cassettes for Lactococcus, Leuconostoc, and Lactobacillus spp. Appl. Environ. Microbiol. 63:4581-4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Loir, Y., S. Nouaille, J. Commissaire, L. Bretigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maassen, C. B., W. J. Boersma, C. Holten-Neelen, E. Claassen, and J. D. Laman. 2003. Growth phase of orally administered Lactobacillus strains differentially affects IgG1/IgG2a ratio for soluble antigens: implications for vaccine development. Vaccine 21:2751-2757. [DOI] [PubMed] [Google Scholar]
- 23.Maassen, C. B., C. Holten-Neelen, F. Balk, M. J. Bak-Glashouwer, R. J. Leer, J. D. Laman, W. J. Boersma, and E. Claassen. 2000. Strain-dependent induction of cytokine profiles in the gut by orally administered Lactobacillus strains. Vaccine 18:2613-2623. [DOI] [PubMed] [Google Scholar]
- 24.Maguin, E., P. Duwat, T. Hege, D. Ehrlich, and A. Gruss. 1992. New thermosensitive plasmid for gram-positive bacteria. J. Bacteriol. 174:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miles, S., R. Fordham, C. Mills, E. Valovirta, and M. Mugford. 2005. A framework for measuring costs to society of IgE-mediated food allergy. Allergy 60:996-1003. [DOI] [PubMed] [Google Scholar]
- 26.Mosmann, T. R., and R. L. Coffman. 1989. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7:145-173. [DOI] [PubMed] [Google Scholar]
- 27.Negroni, L., H. Bernard, G. Clement, J. M. Chatel, P. Brune, Y. Frobert, J. M. Wal, and J. Grassi. 1998. Two-site enzyme immunometric assays for determination of native and denatured beta-lactoglobulin. J. Immunol. Methods 220:25-37. [DOI] [PubMed] [Google Scholar]
- 28.Nouaille, S., L. G. Bermudez-Humaran, K. Adel-Patient, J. Commissaire, A. Gruss, J. M. Wal, V. Azevedo, P. Langella, and J. M. Chatel. 2005. Improvement of bovine beta-lactoglobulin production and secretion by Lactococcus lactis. Braz. J. Med. Biol. Res. 38:353-359. [DOI] [PubMed] [Google Scholar]
- 29.Oozeer, R., J. P. Furet, N. Goupil-Feuillerat, J. Anba, J. Mengaud, and G. Corthier. 2005. Differential activities of four Lactobacillus casei promoters during bacterial transit through the gastrointestinal tracts of human-microbiota-associated mice. Appl. Environ. Microbiol. 71:1356-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oozeer, R., N. Goupil-Feuillerat, C. A. Alpert, M. van de Guchte, J. Anba, J. Mengaud, and G. Corthier. 2002. Lactobacillus casei is able to survive and initiate protein synthesis during its transit in the digestive tract of human flora-associated mice. Appl. Environ. Microbiol. 68:3570-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oozeer, R., D. D. Mater, N. Goupil-Feuillerat, and G. Corthier. 2004. Initiation of protein synthesis by a labeled derivative of the Lactobacillus casei DN-114 001 strain during transit from the stomach to the cecum in mice harboring human microbiota. Appl. Environ. Microbiol. 70:6992-6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porter, E. V., and B. M. Chassy. 1988. Nucleotide sequence of the beta-d-phosphogalactoside galactohydrolase gene of Lactobacillus casei: comparison to analogous pbg genes of other gram-positive organisms. Gene 62:263-276. [DOI] [PubMed] [Google Scholar]
- 33.Posno, M., P. T. Heuvelmans, M. J. van Giezen, B. C. Lokman, R. J. Leer, and P. H. Pouwels. 1991. Complementation of the inability of Lactobacillus strains to utilize d-xylose with d-xylose catabolism-encoding genes of Lactobacillus pentosus. Appl. Environ. Microbiol. 57:2764-2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prioult, G., I. Fliss, and S. Pecquet. 2003. Effect of probiotic bacteria on induction and maintenance of oral tolerance to beta-lactoglobulin in gnotobiotic mice. Clin. Diagn. Lab. Immunol. 10:787-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenfeldt, V., E. Benfeldt, S. D. Nielsen, K. F. Michaelsen, D. L. Jeppesen, N. H. Valerius, and A. Paerregaard. 2003. Effect of probiotic Lactobacillus strains in children with atopic dermatitis. J. Allergy Clin. Immunol. 111:389-395. [DOI] [PubMed] [Google Scholar]
- 36.Seegers, J. F. 2002. Lactobacilli as live vaccine delivery vectors: progress and prospects. Trends Biotechnol. 20:508-515. [DOI] [PubMed] [Google Scholar]
- 37.Steidler, L., W. Hans, L. Schotte, S. Neirynck, F. Obermeier, W. Falk, W. Fiers, and E. Remaut. 2000. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science 289:1352-1355. [DOI] [PubMed] [Google Scholar]
- 38.Sudo, N., S. Sawamura, K. Tanaka, Y. Aiba, C. Kubo, and Y. Koga. 1997. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J. Immunol. 159:1739-1745. [PubMed] [Google Scholar]
- 39.Wal, J. M. 2001. Structure and function of milk allergens. Allergy 56(Suppl. 67):35-38. [PubMed] [Google Scholar]