Abstract
Phagocytic cells form the first line of defense against infections by the human fungal pathogen Candida albicans. Recent in vitro gene expression data suggest that upon phagocytosis by macrophages, C. albicans reprograms its metabolism to convert fatty acids into glucose by inducing the enzymes of the glyoxylate cycle and fatty acid β-oxidation pathway. Here, we asked whether fatty acid β-oxidation, a metabolic pathway localized to peroxisomes, is essential for fungal virulence by constructing two C. albicans double deletion strains: a pex5Δ/pex5Δ mutant, which is disturbed in the import of most peroxisomal enzymes, and a fox2Δ/fox2Δ mutant, which lacks the second enzyme of the β-oxidation pathway. Both mutant strains had strongly reduced β-oxidation activity and, accordingly, were unable to grow on media with fatty acids as a sole carbon source. Surprisingly, only the fox2Δ/fox2Δ mutant, and not the pex5Δ/pex5Δ mutant, displayed strong growth defects on nonfermentable carbon sources other than fatty acids (e.g., acetate, ethanol, or lactate) and showed attenuated virulence in a mouse model for systemic candidiasis. The degree of virulence attenuation of the fox2Δ/fox2Δ mutant was comparable to that of the icl1Δ/icl1Δ mutant, which lacks a functional glyoxylate cycle and also fails to grow on nonfermentable carbon sources. Together, our data suggest that peroxisomal fatty acid β-oxidation is not essential for virulence of C. albicans, implying that the attenuated virulence of the fox2Δ/fox2Δ mutant is largely due to a dysfunctional glyoxylate cycle.
The opportunistic human fungal pathogen Candida albicans is part of the normal microbial flora that colonizes mucosal surfaces, including the gastrointestinal tract and the oral and vaginal mucosa. When the immune system is compromised, superficial colonization by the fungus causes oropharyngeal thrush, vaginitis, and cutaneous infections. However, in severely immunosuppressed patients such as those who have undergone organ transplantation or chemotherapy or are taking broad-spectrum antibiotics, C. albicans may penetrate into deeper tissue and disseminate via the bloodstream, invading organs and causing life-threatening systemic infections (4).
The balance between benign commensal organism and invasive pathogen is largely determined by the activity of the host's innate immune system, and patients with defects in innate immunity are extremely sensitive to systemic C. albicans infections (18, 20, 39, 44). When phagocytic cells of the innate immune system such as macrophages and neutrophils encounter C. albicans cells, they take up the fungus into an intracellular compartment called the phagosome, which fuses with lysosomes to form the phagolysosome. The phagolysosome, characterized by the presence of antimicrobial compounds and reactive oxidative species and a shortage of key nutrients necessary for metabolism, is a hostile environment for C. albicans. However, often, in an ex vivo setting, C. albicans is able to survive its encounter with macrophages. Once inside, the intracellular conditions of the phagolysosome trigger C. albicans to grow as hyphae, which puncture the plasma membrane, thereby destroying the macrophage and thus allowing the fungus to escape.
This outcome illustrates the ability of C. albicans to rapidly adapt to the hostile environment of the macrophage. The response of C. albicans to phagocytosis by macrophages has been studied by genome-wide expression profiling and differential display (15, 21). Both reports revealed that C. albicans reprograms its metabolism in order to cope with nutrient deprivation. In particular, the more extensive studies of Lorenz et al. (15) showed a dramatic upregulation of a specific set of metabolic pathways, i.e., gluconeogenesis, the glyoxylate cycle, and fatty acid β-oxidation, that are all required to convert fatty acids into glucose, implying that inside the macrophage the fungus utilizes lipids for energy production and biosynthesis. Similarly, following exposure to human neutrophils, C. albicans downregulates the glycolytic genes and activates genes encoding the key enzymes of the glyoxylate cycle (ICL1 [isocitrate lyase] and MLS1 [malate synthase]), indicating that the cells face a carbohydrate-poor environment (8, 24). The observation that disruption of ICL1 resulted in a significantly attenuated virulence of C. albicans in a mouse model supports the notion that a functional glyoxylate cycle is required for full virulence in vivo (1, 16). A crucial role for glyoxylate cycle enzymes in virulence has also been shown for Mycobacterium tuberculosis (19), a bacterial pathogen of mammals; for Leptosphaeria maculans (11), Magnaporthe grisae (43), and Stagonospora nodorum (31), plant-pathogenic fungi; and for Rhosococcus fascians (40), a bacterial pathogen of plants. These studies imply that the glyoxylate cycle, and thus lipid metabolism, is a key factor in virulence, in both animal and plant pathogens.
In C. albicans and other fungi, the breakdown of fatty acids takes place only inside peroxisomes, and no mitochondrial β-oxidation is found in these organisms (10). Similarly, in most fungi the key enzymes of the glyoxylate cycle, i.e., ICL1 and MLS1, are localized in peroxisomes (17, 33, 34), although in the yeast S. cerevisiae either one or both enzymes may be extraperoxisomal, depending on the growth conditions (14, 32). Together, these observations suggest an important role for peroxisomes and peroxisomal metabolism in virulence of fungal pathogens.
Peroxisomes are single-membrane-bound organelles that not only contain the enzymes for the β-oxidation of fatty acids but also can be involved in a variety of other metabolic pathways, such as the inactivation of toxic substrates (H2O2-based respiration), the synthesis of etherphospholipids (in mammals), and the breakdown of purines and amino acids (35). Peroxisome biogenesis is a conserved process among eukaryotes, involving the concerted action of at least 32 proteins (peroxins) that are encoded by PEX genes (6, 41). Most of these peroxins function in targeting and import of peroxisomal matrix proteins that are nucleus encoded and synthesized on cytosolic ribosomes. The majority of peroxisomal matrix enzymes contain a type I peroxisomal targeting signal (PTS1), while only a few contain a type II peroxisomal targeting signal (PTS2) (22). Proper targeting and import of PTS1 and PTS2 proteins is mediated by the cycling receptors Pex5p and Pex7p, respectively.
To test whether impaired function of peroxisomes can affect virulence in C. albicans, we constructed a mutant lacking the PTS1 receptor Pex5p. Also, we generated a mutant that has no β-oxidation activity by deleting the FOX2 gene, encoding the second enzyme of the β-oxidation pathway. While both mutant strains were unable to grow on media with fatty acids as sole carbon sources and showed strongly reduced β-oxidation activity in vitro, only the fox2Δ/fox2Δ strain was attenuated in virulence to a degree comparable to that of the icl1Δ/icl1Δ mutant. In vitro growth assays revealed that the fox2Δ/fox2Δ and icl1Δ/icl1Δ mutants could not efficiently utilize two-carbon compounds or lactate, whereas the pex5Δ/pex5Δ mutant showed wild-type growth rates on these carbon sources. These rather unexpected results are discussed in the context of the proposed role of fatty acid metabolism and the glyoxylate shunt in virulence.
MATERIALS AND METHODS
Media and culture conditions.
C. albicans strains were grown at 28°C unless otherwise stated. For routine nonselective culturing of C. albicans strains, 2% Bacto peptone-1% yeast extract-2% glucose-80 μg/ml uridine was used. C. albicans transformants were selected and grown on minimal solid medium containing 0.67% yeast nitrogen base (YNB) without amino acids (Difco), 2% glucose, 80 μg/ml uridine, and amino acids (20 μg/ml arginine and 20 μg/ml histidine) as needed. Plates used for spot assays had the same composition and contained glucose (2%), ethanol (2%), lactate (2%), or sodium acetate (2%, pH 5.0) as a carbon source. The liquid medium used for growing cells for electron microscopy or β-oxidation measurements contained 0.5% potassium phosphate buffer (pH 6.0), 0.3% yeast extract, 0.5% peptone, 0.2% Tween 40, and 0.12% oleic acid as a carbon source. Liquid minimal oleate medium, used for growth curves, contained 0.67% YNB, 0.2% Tween 40, and 0.12% oleate. Before being shifted to one of these media, cells were grown on minimal glucose medium (0.3% glucose, 0.67% YNB) for at least 24 h.
Strains and primers.
Candida albicans strains used in this study are listed in Table 1 and are derivatives of BWP17 (45). Primers are listed in Table 2.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| SC5314 | 45 | |
| BWP17 | ura3Δ::imm434/ura3Δ::imm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG | 45 |
| YKP1 | ura3Δ::imm434/ura3Δ::imm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG PEX5/pex5Δ::ARG4 | This study |
| YKP3 | ura3Δ::imm434/ura3Δ::imm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG pex5Δ::HIS1/pex5Δ::ARG4 | This study |
| CPK1 | ura3Δ::imm434/ura3Δ::URA3 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG pex5Δ::HIS1/pex5Δ::ARG4 | This study |
| CPK3 | ura3Δ::imm434/ura3Δ::URA3::PEX5 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG pex5Δ::HIS1/pex5Δ::ARG4 | This study |
| CPK5 | ura3Δ::imm434/ura3Δ::URA3 his1Δ::hisG/his1Δ::HIS1 arg4Δ::hisG/arg4Δ::ARG4 | This study |
| CPK12 | ura3Δ::imm434/ura3Δ::imm434 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG fox2Δ::ARG4/fox2Δ::HIS1 | This study |
| CEM15 | ura3Δ::imm434/ura3Δ::URA3::FOX2 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG fox2Δ::ARG4/fox2Δ::HIS1 | This study |
| CEM16 | ura3Δ::imm434/ura3Δ::URA3 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG fox2Δ::ARG4/fox2Δ::HIS1 | This study |
| CMD3 | ura3Δ::imm434/ura3Δ his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG icl1Δ::HIS1/icl1Δ::ARG4 | This study |
| CMD8 | ura3Δ::imm434/ura3Δ::URA3 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG icl1Δ::HIS1/icl1Δ::ARG4 | This study |
| CMD6 | ura3Δ::imm434/ura3Δ::URA3::ICL1 his1Δ::hisG/his1Δ::hisG arg4Δ::hisG/arg4Δ::hisG icl1Δ::HIS1/icl1Δ::ARG4 | This study |
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| CaDPex5F | TAATTTTGGATTTTCGAAAATTTGTTATTTTTCTTTCTCCCATATCATTATCAAAGAATGTGGAATTGTGAGCGGATA |
| CaDPex5R | ACAGCTAGAAAAATAATAATTTGCAATTTTGCCATAAATACATACCCTATATACTTAGAAGTTTTCCCAGTCACGACGTT |
| CaPex5F | CCACTTTATCATTTCTCGGTGG |
| CaPex5R | GTTGGTGGTAGGAAGCGTTG |
| pex5intF | CCGCTCGAGCGGCACAATGTCTTGTTGATCTG |
| pex5intR | CGGGATCCCGTCCTGAGTAAAGTTAGACTC |
| URA3INTF | ATCAGTAGCATCATCCTCAGCG |
| URA3INTR | TAGTGATCACTTCTCCTACTCCG |
| pex5-F2 | AGAGGCGTTGATGACTTGGC |
| pex5-R2 | GCCTTGTTTGTCAACTCCTCTA |
| Ca arg3 | TGCCACTGATCCATTGATGGATT |
| Ca arg5 | TTGTGGCATCAATGAAGAACCAG |
| Ca his5 | AAGTTCCAGCAGATGGCGAGTA |
| Ca his3 | GGCCAGTTCCCTTAGATCTAGA |
| HIS1CHR2 | GCGACCAAAGGTGGTATTGACA |
| HIS1CHR | AGGTTTCCCGACTGGAAAGC |
| FOX2-F1 | TTCCTTTTAATCCTTTGAAATATTCTATTTTATTAACTTCAGACATAATACTTTCCTCCCCCCCCCCATTTGTGGAATTGTGAGCGGATA |
| FOX2-R1 | CTTAAAAAGAAAAAAAATAAATACTAACAAAACAAAATAATATAAAAAACTAAATGGCAATTTTACTGCGTTTTCCCAGTCACGACGTT |
| FOX2-F11 | CCCCCTTTTCCTTATTTCCA |
| FOX2-R12 | CAAGACAAACTAACATCAAAACTTCA |
| FOX-F6 | CCCCCCCCATTATGTCTCCAA |
| FOX-F8 | CAGTGTCATCGCTTTCTTTCCA |
| S11-ICL1 | TTTCTTTTTTCCTTCTTTCCTCTATACTTATACCTTTTATTCTAATATAAATTAAAGAATAAACATTAATAATATCTACCGAAGCTTCGTACGCTGCAGGTC |
| S22-ICL1 | CAACAGAATAACTTTCTTATAATTTTACTTACAAATTGTACTACAAAAACAGTTTTCAAAATATAAATAATGTTGAATAATCTGATATCATCGATGAATTCGAG |
| E11-ICL1 | TTCATTCTTTTTTAATACCCTTTTTCTTTTTCTTTTTT |
| E22-ICL1 | TCTCTTTGACGAATAATTGCCAACAGAATAACTTTCTT |
| FP-Icl1 | TCGTTACTCCAAGTGGTCAATG |
| RP-Icl1 | TTTAATGTGCGATGGGTTCA |
| Sac1-Icl1 | AAGAGCTCCCCGTCGAACAAAAGAAAAA |
| ICL1-PstI | TTCTGCAGAAGGCTCAAATTGTTCCCGA |
| Arg2 | AATGGATCAGTGGCACCGGTG |
| Arg4 | GTAGAGTATTTGGTCAGTTATCTGG |
| His4 | GAAATGGCCTCCCCTACCACAG |
| His5 | GGACGAATTGAAGAAAGCTGG |
The PEX5, FOX2, and ICL1 genes were deleted using a PCR-based procedure with primers containing 60- to 70-bp regions of sequence identity to the 5′ and 3′ flanking sequences of the open reading frames (ORFs) (45). The technique involved the construction of two cassettes. The first one, containing the ARG4 auxotrophic marker, was amplified from the plasmid pRS-Arg4*SpeI by using primers CaDPex5F and CaDPex5R (PEX5 disruption), FOX2-F1 and FOX2-R1 (FOX2 disruption), or S11-ICL1 and S22-ICL1 in combination with extension primers E11-ICL1 and E22-ICL1 (ICL1 disruption). The second cassette contained the HIS1 auxotrophic marker, which was amplified on plasmid pGEM-HIS1 with the same primers as used for amplification of the ARG4 cassette. Gene deletions were created through sequential rounds of transformation of strain BWP17 with the disruption cassettes.
To construct pKP5, plasmid pRSArg-N/Ura (45) was digested with BamHI and SpeI, releasing a 1,970-bp fragment encompassing the ARG4 gene. The fragment was cloned between the BamHI and SpeI sites of pAN1 (13), resulting in plasmid pKP5, which was used for complementation of the arginine auxotrophy of BWP17. Plasmid pKP6, used for complementation of the pex5Δ/pex5Δ mutant with the PEX5 gene, was constructed as follows. The wild-type PEX5 sequence from nucleotide −842 to +2374 was amplified from genomic DNA with primers pex5intF and pex5intR, carrying XhoI and BamHI restriction sites, respectively, and ligated into pGEM-T Easy (Promega) vector. The resulting plasmid, pKP1, was digested with XhoI, and the overhanging ends were filled in using Klenow fragment (25). The linearized plasmid was recovered and digested with BamHI, and the fragment encompassing the PEX5 gene was cloned into plasmid pLUBP (23) digested with BamHI and StuI. The resulting plasmid, pKP6, was digested with PacI and integrated into the URA3 locus of strain YKP3 (pex5Δ/pex5Δ), giving rise to the reintegrant strain CPK3 (pex5Δ/pex5Δ + PEX5). The wild-type ICL1 sequence from nucleotide −590 to +2016 was amplified from genomic DNA of strain SC5314 with primers ICL1-PstI and Sac1-ICL1 and ligated into vector pGEM-T (Promega). After sequencing, the 2.6-kb fragment encompassing the ICL1 gene and promoter was cloned into plasmid pLUBP digested with SacI and PstI. The resulting plasmid, pEM311, was digested with PacI and integrated into the URA3 locus of strain CMD3 (icl1Δ/icl1Δ), giving rise to reintegrant strain CMD6 (icl1Δ/icl1Δ + ICL1).
To isolate the FOX2 gene, XhoI- and PstI-digested genomic DNA of C. albicans SC5314 was size fractionated on an agarose gel, and 4- to 5-kb fragments were isolated and cloned into vector pSP73 (Promega). A small library of 8,000 transformants was screened with a 2.6-kb FOX2 probe that was generated by PCR on genomic DNA by using primers FOX-F6 and FOX-R8. Four independent transformants that contained a 4.5-kb XhoI-PstI fragment encompassing the FOX2 ORF, 1.6-kb 5′ sequences, and 0.3 kb of 3′ sequences were identified. One of these plasmids (pEM501) was digested with XhoI, blunt ended, and digested with PstI. The resulting 4.5-kb FOX2 fragment was inserted into pLUBP digested with StuI and PstI to give plasmid pEM503. The FOX2 reintegrant strain CEM15 was constructed by integrating XhoI-digested pEM503 into strain CPK12 (fox2Δ/fox2Δ).
To create prototrophic strains of pex5Δ/pex5Δ (YKP3), fox2Δ/fox2Δ (CPK12), and icl1Δ/icl1Δ (CMD3), the strains were transformed with PacI- or XhoI-digested pLUBP, resulting in strains CPK1, CEM16, and CMD8, respectively. Finally, a prototrophic BWP17 strain (CPK5) was constructed by sequential transformation of strain BWP17 with plasmids pLUBP, pKP5, and pGEM-HIS1 digested with PacI, NotI, and PstI, respectively. All strains were verified by PCR and Southern hybridization.
Southern analysis.
Southern blotting was carried out with standard protocols (25), using randomly primed probes. The PEX5 probe was a 0.7-kb SalI-BamHI fragment of plasmid pKP1 comprising 0.1 kb of the PEX5 ORF and 0.6 kb of 3′sequences. The FOX2 probe was a 1.7-kb XbaI-ClaI fragment comprising 0.5-kb promoter sequences and 1.2 kb of the FOX2 ORF. Radioactive signals were revealed by exposure to Kodak Bio Max MR film (Amersham Biosciences).
Transformation.
C. albicans was transformed using a modified lithium acetate protocol (42). The heat shock was carried out at 44°C for 15 min.
Fatty acid β-oxidation measurements.
Measurements of β-oxidation in intact cells were performed essentially as described by van Roermund et al. (37) except that the cells were resuspended at an optical density (OD) at 600 nm of 1 and the incubations with radiolabeled fatty acids were allowed to proceed for 45 min. The β-oxidation capacity of wild-type cells grown on oleate in each experiment was taken as a reference (100%).
Antibodies.
Polyclonal antibodies directed against S. cerevisiae 3-ketoacyl coenzyme A (3-ketoacyl-CoA) thiolase or catalase were used in this study. Both antibodies cross-react specifically with the corresponding peroxisomal proteins in C. albicans (our unpublished results).
Electron microscopy.
Oleate-induced cells were fixed with 2% (wt/vol) formaldehyde, and ultrathin sections were prepared as described previously (9). Immunolabeling was performed using antibodies directed against S. cerevisiae 3-ketoacyl-CoA thiolase or catalase and gold-conjugated protein A.
Virulence studies.
Virulence assays were performed at the University of Aberdeen, using a murine tail vein injection model. Four independent experiments were carried out with 6 to 12 female BALB/c mice in each injection group. All C. albicans strains, except for the icl1Δ/icl1Δ mutant and icl1Δ/icl1Δ reintegrant, were tested at least twice. Strains were grown overnight at 30°C in 0.1% neopeptone-0.4% glucose-0.1% yeast extract to early stationary phase (2 × 107 CFU/ml ± 0.3 × 107 CFU/ml). Cells were centrifuged (10 min at 2,500 × g), washed twice, and resuspended in sterile saline. The OD of the final suspension was adjusted according to a predetermined calibration curve of OD versus CFU per ml to yield suspensions of cells calculated to give suitable yeast inoculum doses for intravenous injection in 100-μl volumes for mice weighing 20 g. Actual CFU/ml in inoculum suspensions were determined by viable counting procedures and ranged from 1.6 × 104 to 4.5 × 104 CFU per gram body weight. Animals were observed and weighed daily for a period of 28 days after challenge. Animals that lost more than 20% of their body weight or otherwise showed signs of irrecoverably severe disease were humanely killed and recorded as having died on the following day. Tissue burdens were assessed on the day of demise of the animal. For all animals, the left and right kidneys and brain were removed postmortem, weighed, and homogenized in saline, and tissue burdens of fungi were determined by viable counts of the homogenate. For the purpose of calculating mean burdens, when tissues were negative for recovery of C. albicans they were recorded as containing 0.5 log10 CFU/g below the detection threshold, i.e., 1.8 log10 CFU/g for kidney and 1.3 log10 CFU/g for brain.
Statistical analysis.
Survival data were analyzed by log rank/Kaplan-Meier statistics. Tissue burden data were analyzed by the Mann-Whitney U test.
RESULTS
Identification of the C. albicans PEX5 and FOX2 genes.
In order to identify the genes encoding CaPex5p and CaFox2p, a BLASTp search with S. cerevisiae Pex5p and Fox2p was performed. This resulted in the identification of a single Pex5-like open reading frame (orf19.5640), encoding a protein of 593 residues, and a single Fox2-like open reading frame (orf19.1809), encoding a 907-amino-acid protein. Sequence alignment of CaPex5p and CaFox2p with their S. cerevisiae homologues revealed overall identities of 41% and 52%, respectively. Accordingly, these ORFs were named CaPEX5 and CaFOX2. The PEX5 gene was amplified by PCR with genomic DNA prepared from the wild-type strain SC5314, using gene-specific oligonucleotides. The clone was sequenced to confirm its identity. Because of difficulties with PCR amplification of the 5′ end of the FOX2 gene, a small genomic library was constructed (see Materials and Methods), and a single clone containing a 4.6-kb fragment was isolated, encompassing 1.6-kb upstream sequences, the complete FOX2 ORF, and 0.3-kb downstream sequences.
Construction of C. albicans pex5 and fox2 null mutants by targeted gene disruption.
To investigate the roles of CaPex5p and CaFox2p in C. albicans virulence, we created strains in which both copies of either the PEX5 or the FOX2 gene were deleted (pex5Δ/pex5Δ and fox2Δ/fox2Δ) by using a PCR-based gene disruption procedure described by Wilson et al. (45). Correct integration of the ARG4 and HIS1 cassettes at the PEX5 and FOX2 loci was verified by PCR (results not shown) and Southern blot analysis (Fig. 1).
FIG. 1.
Southern blot analysis of the constructed strains. Shown are the genomic configurations of the PEX5 and FOX2 wild-type loci, the deleted derivatives, and the reintegrants at the URA3 locus. Genomic DNA was digested with XbaI and analyzed by Southern blotting using the indicated probes (dotted lines). The strains used were the prototrophic wild-type strain CPK5 (PEX5/PEX5 and FOX2/FOX2), CPK1 (pex5Δ::ARG4/pex5Δ::HIS1), CPK3 (pex5Δ::ARG4/pex5Δ::HIS1 + PEX5), CEM16 (fox2Δ::ARG4/fox2Δ::HIS1), and CEM 15 (fox2Δ::ARG4/fox2Δ::HIS1 + FOX2). Due to allelic variation, resulting in loss of the XbaI site downstream of the FOX2 gene, two bands are visible for the wild-type strain CPK5 probed with the FOX2 probe.
Recently, it was demonstrated that different genomic positions of the URA3 gene might result in different levels of URA3 gene expression, thereby altering the virulence potential of C. albicans strains (2, 5). To avoid positional effects on URA3 expression, we reintroduced the URA3 gene at a common locus in all strains. For this, we used vectors based on the pLUBP plasmid (23), which contains the complete URA3 gene and the flanking IRO1 gene. Vectors were integrated at the ura3 locus, thereby restoring Ura3 prototrophy and reintroducing the wild-type copy of the deleted gene at the same locus (Fig. 1). Ura3 prototrophs of the double-deletion strains were obtained by integration of an “empty,” linearized pLUBP vector. The correct integration of plasmids was confirmed by PCR and Southern analysis (results not shown).
Characterization of the pex5 and fox2 null mutants by immunoelectron microscopy.
Previous studies with other organisms have shown that deletion of the PEX5 gene, encoding the receptor Pex5p, results in mislocalization of peroxisomal proteins containing a PTS1, while PTS2 proteins are properly localized.
To determine whether the Capex5Δ/pex5Δ strain also shows a PTS1-dependent mislocalization of peroxisomal matrix proteins, we performed immunoelectron microscopy on oleate-induced C. albicans cells with antibodies directed against S. cerevisiae thiolase (a PTS2 protein) and catalase (a PTS1 protein). Western blot analysis of total lysates of wild-type C. albicans cells showed that both antibodies specifically cross-react with the corresponding proteins in C. albicans (data not shown). Immunocytochemistry of the pex5Δ/pex5Δ strain revealed mislocalization of catalase as indicated by significant labeling of the cytosol and nucleus, whereas thiolase was confined to membrane structures (Fig. 2). These results are in line with previous studies of S. cerevisiae, which showed that in pex5Δ/pex5Δ cells, thiolase could still be imported in peroxisomal membrane structures lacking all of the PTS1-containing matrix proteins (36). In wild-type C. albicans cells, the anticatalase and antithiolase antibodies only labeled peroxisomal profiles, confirming the specificity of the antibodies used. Catalase and thiolase labeling in the complemented pex5Δ/pex5Δ strain (pex5Δ/pex5Δ + PEX5) was indistinguishable from that in wild-type cells, indicating that PTS1 import was fully restored in the revertant.
FIG. 2.
Characterization of the pex5Δ/pex5Δ and fox2Δ/fox2Δ strains by immunoelectron microscopy. Oleate-induced wild-type cells (A and E), pex5Δ/pex5Δ cells (B and F), fox2Δ/fox2Δ cells (C and G), or complemented pex5Δ/pex5Δ cells (D and H) were fixed and prepared for immunocytochemistry. Cryosections were incubated with antibodies directed against catalase (A to D) or thiolase (E to H), and antigens were visualized with immunogold particles conjugated to protein A. P, peroxisome, M, mitochondrion, N, nucleus. Bars, 0.5 μm.
As expected, deletion of the FOX2 gene, encoding the bi- or multifunctional enzyme (Fox2p), did not influence the transport of peroxisomal proteins, as indicated by the strong labeling of the peroxisomal profiles with both anticatalase and antithiolase. However, peroxisomes in fox2Δ/fox2Δ cells appeared to be larger and more irregular in structure than those in wild-type cells (or the complemented fox2Δ/fox2Δ strain [data not shown]). Such a phenotype has been observed before in yeast and mammalian mutants with deficiencies in peroxisomal fatty acid β-oxidation and has been attributed to defects in peroxisome proliferation (29, 38).
The pex5 and fox2 null mutants are unable to grow on oleic acid-containing medium.
S. cerevisiae mutants with defects in peroxisome biogenesis (e.g., pex5Δ) or peroxisomal β-oxidation (e.g., fox2Δ) cannot utilize fatty acids as the sole carbon source (10). Therefore, we tested the ability of the C. albicans pex5Δ/pex5Δ and fox2Δ/fox2Δ null mutants to grow on minimal medium containing oleic acid as the exclusive carbon source (Fig. 3). No increase in optical density was observed for the pex5Δ/pex5Δ or the fox2Δ/fox2Δ strain during the time course of the experiment (24 h). In contrast, the strains with the reintroduced PEX5 or FOX2 gene showed growth rates comparable to that of the wild-type strain. All strains grew equally well on medium containing glucose as the carbon source (see Fig. 6). From these experiments, we conclude that both deletion strains cannot use oleic acid as the sole carbon source and that the oleic acid-nonutilizing phenotype was fully suppressed in the complemented null strains.
FIG. 3.
The pex5Δ/pex5Δ and fox2Δ/fox2Δ mutants are unable to grow on oleic acid-containing medium. Strains were pregrown on minimal medium containing 0.3% glucose and inoculated at an OD at 600 nm of 0.1 in minimal oleate medium. Growth was monitored over a period of 24 h by measuring the optical density of the cultures.
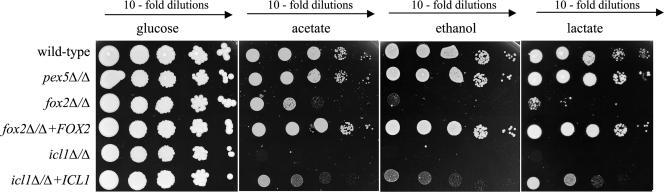
FIG. 6.
Growth characteristics of wild-type and mutant strains on different carbon sources. Cells were pregrown on medium containing 0.3% glucose, washed, resuspended to a concentration of about 2.7 × 107 cells/ml, and serially diluted (1:10 dilutions). Five microliters of each dilution was spotted onto agar plates. The pictures were taken after 3 days (glucose) or five days (acetate, ethanol, and lactate) of incubation at 28°C.
The pex5 and fox2 null mutants have strongly reduced fatty acid β-oxidation activity.
The above results suggested that the pex5Δ/pex5Δ and fox2Δ/fox2Δ strains could not metabolize oleic acid. To directly determine their capacity to degrade fatty acids, we measured the oxidation of 1-14C-labeled oleic acid in these strains. As shown in Fig. 4, oxidation of [1-14C]oleic acid in the pex5Δ/pex5Δ mutant was strongly reduced, to about 5% of the β-oxidation activity present in wild-type cells. In the strain lacking Fox2p, β-oxidation activity dropped to background levels (∼0.2% of the activity in wild-type cells). Complementation of the deletion strains with the corresponding wild-type gene almost completely restored fatty acid oxidation to wild-type levels. Similar results were found when the strains were tested for their ability to oxidize short-chain fatty acids (octanoic acid) (data not shown). Together, these data indicate that peroxisomal fatty acid β-oxidation of both short- and long chain fatty acids is strongly reduced in the pex5Δ/pex5Δ and fox2Δ/fox2Δ strains. Furthermore, these results support the notion that there is a single FOX2 gene encoding the peroxisomal multifunctional enzyme in C. albicans.
FIG. 4.
The pex5Δ/pex5Δ and fox2Δ/fox2Δ mutants have strongly reduced fatty acid β-oxidation activity. Strains were induced on oleate, and β-oxidation activity was measured as described in Materials and Methods. β-Oxidation rates are expressed as percentages of the activity measured in the prototrophic wild-type (WT) strain BWP17. Error bars indicate standard deviations.
The fox2 null mutant, but not the pex5 null mutant, is significantly attenuated in virulence.
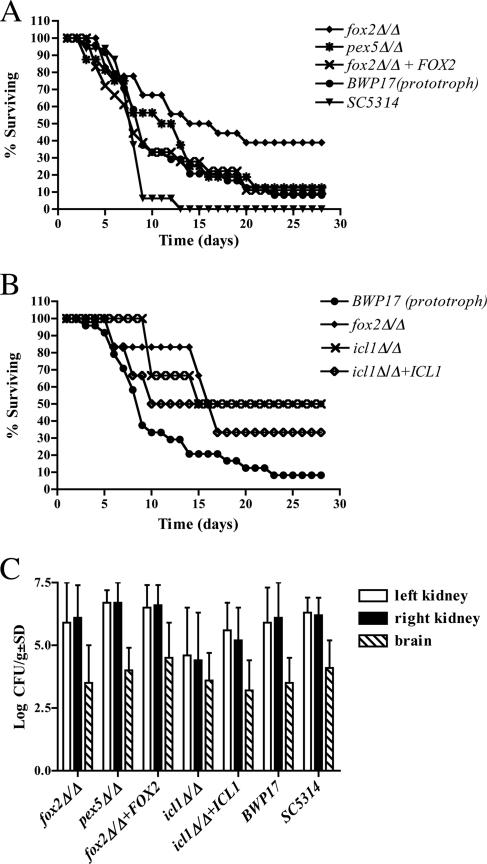
To assess the virulence of the pex5Δ/pex5Δ and fox2Δ/fox2Δ strains, four separate experiments were performed in which groups of 12 (first experiment) or 6 (second, third, and fourth experiments) female BALB/c mice were infected intravenously with a challenge dose in the range of 1.6 × 104 to 4.5 × 104 CFU/g body weight. As controls, the complemented derivatives (fox2Δ/fox2Δ + FOX2 and pex5Δ/pex5Δ + PEX5), the prototrophic BWP17, and the clinical isolate SC5314 were used. In Fig. 5A the combined data from four separate experiments are summarized. The survival curves show that all strains derived from BWP17 (including the prototrophic BWP17) are slightly attenuated in virulence relative to the wild-type strain SC5314. The reason for the reduced virulence of BWP17-derived strains is currently not known, but it may be related to the observation that this strain does not have a full-length copy of chromosome 5 (27). Nevertheless, survival of the BWP17-derived pex5Δ/pex5Δ mutant was not significantly different from that of the prototrophic BWP17 strain (P = 0.655) or the pex5Δ/pex5Δ reintegrant (data not shown). A small but significant difference in survival between the fox2Δ/fox2Δ mutant and BWP17 (P = 0.027) was observed. Reintroduction of the FOX2 gene completely suppressed this virulence defect, indicating that FOX2 function is required for full virulence of C. albicans. Analysis of left and right kidney burdens and brain burdens of the infected mice showed no significant difference between the six strains tested (Fig. 5C and data not shown).
FIG. 5.
Virulence of the pex5Δ/pex5Δ, fox2Δ/fox2Δ, and icl1Δ/icl1Δ mutants in a mouse model for systemic candidiasis. (A) Survival of the pex5Δ/pex5Δ, fox2Δ/fox2Δ, and fox2Δ/fox2Δ reintegrant was compared to that of the prototrophic BWP17 strain and the clinical isolate SC5314. BALB/c mice were infected intravenously with a challenge dose in the range of 1.6 × 104 to 4.5 × 104 CFU/g body weight. The combined data from four separate experiments are shown. (B) Comparison of the virulence of the icl1Δ/icl1Δ mutant and the icl1Δ/icl1Δ reintegrant with that of the fox2Δ/fox2Δ mutant. As a control, the survival curve of the prototrophic parental strain BWP17 is shown. (C) Mean tissue burdens in brain, left kidney, and right kidney for mice infected with each of the seven C. albicans strains. Error bars indicate standard deviations.
The fox2 and icl1 null mutants display similar degrees of virulence attenuation.
Based on transcription profiling studies, it has been suggested that the ability of C. albicans to convert fatty acids into glucose, a metabolic process that requires the glyoxylate cycle, may contribute to its virulence in vivo (15, 16). Indeed, inactivation of the ICL1 gene, encoding a key enzyme of the glyoxylate cycle, attenuated virulence of C. albicans (1, 16). However, since the ICL1 gene deletions were made in different strain backgrounds by different procedures, a direct comparison with our BWP17-derived fox2Δ/fox2Δ strain was not possible. Therefore, we generated an icl1 null mutant in BWP17 and determined the effect of inactivating ICL1 on C. albicans virulence (Fig. 5B). Deletion of ICL1 partially but significantly attenuated virulence (P = 0.027), confirming previous results (1, 16). This virulence defect was suppressed, albeit incompletely, by reintroduction of the ICL1 gene. Interestingly, the icl1 null mutant displayed a similar degree of virulence attenuation as the fox2Δ/fox2Δ mutant. At first sight these results seem to be in concordance: icl1Δ/icl1Δ cells (16) and fox2Δ/fox2Δ cells (Fig. 3) cannot grow in vitro on fatty acids and show similar reductions in virulence, suggesting that the capacity to β-oxidize fatty acids plays a role in C. albicans virulence. However, our results with the pex5Δ/pex5Δ mutant do not support this conclusion: pex5Δ/pex5Δ cells also have a strongly reduced fatty acid β-oxidation activity and, accordingly, do not grow on media with fatty acids, but virulence is not affected in this mutant. We therefore investigated the growth behavior of all three mutant strains on nonfermentable carbon sources other than fatty acids.
The fox2Δ/fox2Δ and icl1Δ/icl1Δ mutants, but not the pex5Δ/pex5Δ mutant, display severe growth defects on nonfermentable carbon sources other than fatty acids.
Serial dilutions of cells pregrown in 0.3% glucose medium were spotted onto plates containing acetate (C2), ethanol (C2), lactate (C3), or glucose (C6) as a carbon source (Fig. 6). As expected, the icl1Δ/icl1Δ mutant did not grow on acetate or ethanol and, surprisingly, also did not grow on lactate. The growth behavior of the fox2Δ/fox2Δ strain was remarkably similar to that of the icl1Δ/icl1Δ strain: it had strongly reduced growth on acetate or lactate and virtually no growth on ethanol. These phenotypes were suppressed completely (FOX2) or partially (ICL1) by introduction of the corresponding wild-type gene. In contrast, the pex5Δ/pex5Δ mutant exhibited growth rates comparable to those of wild-type cells on all three nonfermentable carbon sources. Furthermore, all strains grew equally well on glucose. These results indicate that the fox2Δ/fox2Δ mutant, like the icl1Δ/icl1Δ mutant, cannot efficiently utilize nonfermentable carbon sources such as acetate, ethanol, or lactate, a phenotype that is not observed for the pex5Δ/pex5Δ mutant.
DISCUSSION
Recent studies have suggested that the ability of C. albicans to convert fatty acids into glucose may contribute to its virulence in vivo (15, 16, 21). Three metabolic pathways are essential for this conversion: (i) fatty acid β-oxidation, a metabolic pathway that is localized in peroxisomes in all yeasts and mediates the breakdown of fatty acids to acetyl-CoA (C2) units; (ii) the glyoxylate cycle, a pathway that allows the conversion of acetyl-CoA units to C4 compounds; and (iii) gluconeogenesis, for the synthesis of C6 compounds (e.g., glucose) from C4 units. Here we used two C. albicans mutants, pex5Δ/pex5Δ and fox2Δ/fox2Δ, to directly address the role of peroxisomal function and peroxisomal fatty acid β-oxidation in the virulence of this organism. The pex5Δ/pex5Δ mutant is disturbed in peroxisome biogenesis and specifically mislocalizes PTS1-containing proteins to the cytosol (Fig. 2). As a consequence, fatty acid β-oxidation is strongly reduced, and pex5Δ/pex5Δ cells cannot grow on media with oleic acid as the sole carbon source (Fig. 3 and 4). The fox2Δ/fox2Δ mutant lacks in vitro β-oxidation activity and, accordingly, does not grow on oleic acid-containing medium (Fig. 3 and 4). These data and the Southern blot analyses show that C. albicans contains a single PEX5 gene and a single FOX2 gene.
Despite the inability of both mutants to grow on fatty acids as the sole carbon and energy source, virulence assays in a mouse model for systemic candidiasis revealed that only the fox2Δ/fox2Δ strain is significantly attenuated in virulence (Fig. 5). How can the difference between the fox2Δ/fox2Δ mutant and the pex5Δ/pex5Δ mutant be explained? Direct comparison of the two mutant strains with the icl1Δ/icl1Δ glyoxylate cycle mutant with respect to their growth and virulence phenotypes revealed a striking similarity between the fox2Δ/fox2Δ mutant and the icl1Δ/icl1Δ mutant: both mutant strains could not efficiently utilize nonfermentable carbon sources such as acetate, ethanol, or lactate, and in a mouse model for systemic candidiasis the two mutant strains displayed similar degrees of virulence attenuation (Fig. 5 and 6). In contrast, the pex5Δ/pex5Δ mutant showed wild-type growth rates on these nonfermentable carbon sources and, as mentioned above, was not attenuated in virulence. These data suggest a strong correlation between in vivo virulence attenuation and the inability of mutant cells to grow in vitro on nonfermentable carbon sources other than fatty acids, and they imply that in the fox2Δ/fox2Δ mutant the glyoxylate cycle does not function optimally.
In C. albicans, like in most fungi, the key enzymes of the glyoxylate shunt are localized in the peroxisomal matrix, and their import into the organelles is dependent on the PTS1 receptor Pex5p (unpublished data). The fact that growth of the pex5Δ/pex5Δ strain on two-carbon compounds is not disturbed indicates that the glyoxylate cycle functions equally well in the peroxisome and in the cytosol and implies that there is no requirement for a peroxisomal compartmentalization of this metabolic pathway. Why the glyoxylate cycle is deficient in the fox2Δ/fox2Δ strain remains to be investigated.
Our observations are rather unexpected in light of the accumulating data that suggest an important role of the fatty acid β-oxidation pathway in bacterial (19) and fungal (15, 16, 21) virulence. The persistence defect of M. tuberculosis isocitrate lyase mutants is thought to be due to an inability to utilize fatty acids as the carbon source when the bacteria are in a lipid-rich environment of macrophages (19). A similar suggestion has been put forward to explain the reduced virulence of a C. albicans icl1Δ/icl1Δ mutant (16). In support of this, Lorenz et al. (15) found that upon ingestion by macrophages, C. albicans induces most of the enzymes required for the breakdown of fatty acids. Our results suggest that fatty acids are not the exclusive source of acetyl-CoA within the phagolysosome. Therefore, the question arises as to which other compounds could serve as precursors of these C2 units. Our in vitro growth assays and the transcription profiling data of Lorenz et al. provide a possible answer. Phagocytosis of both S. cerevisiae and C. albicans by macrophages results in a strongly increased expression of ACS1 (acetyl-CoA synthase) (8.7- and 6.1-fold induced, respectively) and YAT1 (carnitine acetyltransferase) (36.6- and 20.4-fold induced, respectively). In S. cerevisiae, YAT1 is even more highly induced than the glyoxylate cycle genes (16). ACS1 is also upregulated together with the glyoxylate cycle genes ICL1 and MLS1 upon incubation of C. albicans with neutrophils (8). The ACS1 product, a cytosolic enzyme in yeasts (12, 28), catalyzes the ATP-dependent activation of acetate to acetyl-CoA. The cytosolic acetyl-CoA is either shuttled into mitochondria, requiring the carnitine acetyltransferase YAT1 localized in the outer mitochondrial membrane (26), or serves as a building block for lipid synthesis (7). However, it is unlikely that acetate is present in sufficiently high concentration inside the phagolysosome to sustain growth of C. albicans.
A more likely explanation is that the intracellular acetate is the product of lactate degradation (Fig. 7). Among the C. albicans genes that are most highly induced in macrophages are JEN1 (5.5-fold induced) and its close homolog JEN2 (orf19.5307) (159.5-fold induced), encoding lactate permeases, and CYB2 (15.1-fold induced), coding for l-lactate dehydrogenase (30). l-Lactate is abundantly present in the human body and is produced at high rates by red blood cells, by brain, and by muscle (3). Lactate taken up by C. albicans is oxidized to pyruvate by lactate dehydrogenase. The observed induction of the cytosolic pyruvate decarboxylase pathway (involving pyruvate decarboxylase [PDC12] [1.4-fold induced] and acetaldehyde dehydrogenase [orf19.6306] [7.6-fold induced]) and the downregulation of the mitochondrial pyruvate dehydrogenase complex (PDA1 and PDB1) (4- to 5-fold repressed) suggest that the lactate-derived pyruvate is converted to acetate in the cytosol. Strikingly, the enzymes required for lactate utilization and production of cytosolic acetyl-CoA are strongly repressed (>10-fold) in C. albicans challenged with fatty acids only (our unpublished microarray data). These data, together with the observation that lactate induces the expression of the lactate permease gene JEN1 (30), may point to the presence of lactate in the microenvironment of the phagosome, the utilization of which may contribute to the survival of C. albicans. The requirement for the glyoxylate bypass when lactate (a C3 compound) is present as a carbon and energy source is rather unexpected, since the lactate-derived pyruvate can be converted to oxaloacetate (C4) by pyruvate carboxylase (PYC2) (Fig. 7). However, our in vitro growth assays show that the C. albicans icl1Δ/icl1Δ mutant cannot grow on lactate, suggesting that the pyruvate decarboxylase pathway is the preferred route under these conditions and implying a key role for the glyoxylate shunt to synthesize C4 carbon compounds for gluconeogenesis.
FIG. 7.
Schematic drawing of metabolic routes possibly involved in lactate utilization in C. albicans. The figure shows the lactate transporters JEN1 and JEN2, the l-lactate dehydrogenase CYB2, the pyruvate decarboxylase pathway or “pyruvate dehydrogenase bypass” (highlighted with thick arrows), the tricarboxylic acid cycle, and the glyoxylate bypass. Other abbreviations: PDC, pyruvate decarboxylase; PYC, pyruvate carboxylase; PDA/B, pyruvate dehydrogenase complex; ALD, acetaldehyde dehydrogenase; ACS, acetyl-CoA synthase; YAT, carnitine acetyltransferase; ICL, isocitrate lyase; MLS, malate synthase; MDH, malate dehydrogenase. For details see text.
Several studies have now shown that following phagocytosis by macrophages or neutrophils, C. albicans cells downregulate glycolytic genes and activate glyoxylate cycle and gluconeogenic genes, allowing the assimilation of two-carbon compounds (1, 8, 15, 16, 21). The role of the glyoxylate cycle and gluconeogenesis in C. albicans pathogenesis has been substantiated by analysis of deletion mutants lacking the key enzymes of these metabolic pathways (1, 16; this study). Our data indicate that peroxisomal fatty acid β-oxidation, despite the strong induction of the enzymes of this pathway after phagocytosis by macrophages, does not significantly contribute to the ability of C. albicans to survive in vivo. Furthermore, since the glyoxylate cycle was shown to be essential for fungal virulence, our data suggest that, in vivo, the acetyl-CoA required to feed the glyoxylate cycle is derived not from the breakdown of fatty acids but from other nonfermentable carbon sources such as acetate or lactate.
Acknowledgments
We thank Aaron Mitchell (Columbia University, New York, N.Y.) for providing strains and plasmids, William Fonzi (Georgetown University, Washington, D.C.) for plasmid pLUBP, and Jürgen Wendland (Friedrich-Schiller-University, Jena, Germany) for the pFA modules. We are grateful to Matthijs Dekkers for help with the construction of the icl1Δ/icl1Δ strain and to Rob Benne and Frans Hochstenbach for valuable comments and suggestions.
This work was supported by grants from the Academic Medical Center and the European Community (QLG2-CT-2001-01663).
Footnotes
Published ahead of print on 8 September 2006.
REFERENCES
- 1.Barelle, C. J., C. L. Priest, D. M. Maccallum, N. A. Gow, F. C. Odds, and A. J. Brown. 2006. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell. Microbiol. 8:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brand, A., D. M. MacCallum, A. J. Brown, N. A. Gow, and F. C. Odds. 2004. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot. Cell 3:900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchalter, S. E., M. R. Crain, and R. Kreisberg. 1989. Regulation of lactate metabolism in vivo. Diabetes Metab. Rev. 5:379-391. [DOI] [PubMed] [Google Scholar]
- 4.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, S., M. H. Nguyen, Z. Zhang, H. Jia, M. Handfield, and C. J. Clancy. 2003. Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect. Immun. 71:6101-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Distel, B., R. Erdmann, S. J. Gould, G. Blobel, D. I. Crane, J. M. Cregg, G. Dodt, Y. Fujiki, J. M. Goodman, W. W. Just, J. A. Kiel, W. H. Kunau, P. B. Lazarow, G. P. Mannaerts, H. W. Moser, T. Osumi, R. A. Rachubinski, A. Roscher, S. Subramani, H. F. Tabak, T. Tsukamoto, D. Valle, I. van der Klei, P. P. van Veldhoven, and M. Veenhuis. 1996. A unified nomenclature for peroxisome biogenesis factors. J. Cell Biol. 135:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flikweert, M. T., M. de Swaaf, J. P. van Dijken, and J. T. Pronk. 1999. Growth requirements of pyruvate-decarboxylase-negative Saccharomyces cerevisiae. FEMS Microbiol. Lett. 174:73-79. [DOI] [PubMed] [Google Scholar]
- 8.Fradin, C., P. De Groot, D. MacCallum, M. Schaller, F. Klis, F. C. Odds, and B. Hube. 2005. Granulocytes govern the transcriptional response, morphology and proliferation of Candida albicans in human blood. Mol. Microbiol. 56:397-415. [DOI] [PubMed] [Google Scholar]
- 9.Hettema, E. H., W. Girzalsky, M. van den Berg, R. Erdmann, and B. Distel. 2000. Saccharomyces cerevisiae pex3p and pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiltunen, J. K., A. M. Mursula, H. Rottensteiner, R. K. Wierenga, A. J. Kastaniotis, and A. Gurvitz. 2003. The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol. Rev. 27:35-64. [DOI] [PubMed] [Google Scholar]
- 11.Idnurm, A., and B. J. Howlett. 2002. Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryot. Cell 1:719-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kispal, G., J. Cseko, I. Alkonyi, and A. Sandor. 1991. Isolation and characterization of carnitine acetyltransferase from S. cerevisiae. Biochim. Biophys. Acta 1085:217-222. [DOI] [PubMed] [Google Scholar]
- 13.Klein, A. T., P. Barnett, G. Bottger, D. Konings, H. F. Tabak, and B. Distel. 2001. Recognition of peroxisomal targeting signal type 1 by the import receptor Pex5p. J. Biol. Chem. 276:15034-15041. [DOI] [PubMed] [Google Scholar]
- 14.Kunze, M., F. Kragler, M. Binder, A. Hartig, and A. Gurvitz. 2002. Targeting of malate synthase 1 to the peroxisomes of Saccharomyces cerevisiae cells depends on growth on oleic acid medium. Eur. J. Biochem. 269:915-922. [DOI] [PubMed] [Google Scholar]
- 15.Lorenz, M. C., J. A. Bender, and G. R. Fink. 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot. Cell 3:1076-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz, M. C., and G. R. Fink. 2001. The glyoxylate cycle is required for fungal virulence. Nature 412:83-86. [DOI] [PubMed] [Google Scholar]
- 17.Maeting, I., G. Schmidt, H. Sahm, J. L. Revuelta, Y. D. Stierhof, and K. P. Stahmann. 1999. Isocitrate lyase of Ashbya gossypii—transcriptional regulation and peroxisomal localization. FEBS Lett. 444:15-21. [DOI] [PubMed] [Google Scholar]
- 18.Mansour, M. K., and S. M. Levitz. 2002. Interactions of fungi with phagocytes. Curr. Opin. Microbiol. 5:359-365. [DOI] [PubMed] [Google Scholar]
- 19.McKinney, J. D., K. Honer zu Bentrup, E. J. Munoz-Elias, A. Miczak, B. Chen, W. T. Chan, D. Swenson, J. C. Sacchettini, W. R. Jacobs, Jr., and D. G. Russell. 2000. Persistence of Mycobacterium tuberculosis in macrophages and mice requires the glyoxylate shunt enzyme isocitrate lyase. Nature 406:735-738. [DOI] [PubMed] [Google Scholar]
- 20.Odds, F. C. 1988. Candida and candidiosis: a review and bibliography, 2nd ed. Ballière Tindall, London, United Kingdom.
- 21.Prigneau, O., A. Porta, J. A. Poudrier, S. Colonna-Romano, T. Noel, and B. Maresca. 2003. Genes involved in beta-oxidation, energy metabolism and glyoxylate cycle are induced by Candida albicans during macrophage infection. Yeast 20:723-730. [DOI] [PubMed] [Google Scholar]
- 22.Purdue, P. E., and P. B. Lazarow. 2001. Peroxisome biogenesis. Annu. Rev. Cell Dev. Biol. 17:701-752. [DOI] [PubMed] [Google Scholar]
- 23.Ramón, A. M., and W. A. Fonzi. 2003. Diverged binding specificity of Rim101p, the Candida albicans ortholog of PacC. Eukaryot. Cell 2:718-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin-Bejerano, I., I. Fraser, P. Grisafi, and G. R. Fink. 2003. Phagocytosis by neutrophils induces an amino acid deprivation response in Saccharomyces cerevisiae and Candida albicans. Proc. Natl. Acad. Sci. USA 100:11007-11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 26.Schmalix, W., and W. Bandlow. 1993. The ethanol-inducible YAT1 gene from yeast encodes a presumptive mitochondrial outer carnitine acetyltransferase. J. Biol. Chem. 268:27428-27439. [PubMed] [Google Scholar]
- 27.Selmecki, A., S. Bergmann, and J. Berman. 2005. Comparative genome hybridization reveals widespread aneuploidy in Candida albicans laboratory strains. Mol. Microbiol. 55:1553-1565. [DOI] [PubMed] [Google Scholar]
- 28.Sheridan, R., C. Ratledge, and P. A. Chalk. 1990. Pathways to acetyl-CoA formation in Candida albicans. FEMS Microbiol. Lett. 57:165-169. [DOI] [PubMed] [Google Scholar]
- 29.Smith, J. J., T. W. Brown, G. A. Eitzen, and R. A. Rachubinski. 2000. Regulation of peroxisome size and number by fatty acid beta-oxidation in the yeast Yarrowia lipolytica. J. Biol. Chem. 275:20168-20178. [DOI] [PubMed] [Google Scholar]
- 30.Soares-Silva, I., S. Paiva, P. Kotter, K. D. Entian, and M. Casal. 2004. The disruption of JEN1 from Candida albicans impairs the transport of lactate. Mol. Membr. Biol. 21:403-411. [DOI] [PubMed] [Google Scholar]
- 31.Solomon, P. S., R. C. Lee, T. J. Wilson, and R. P. Oliver. 2004. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol. Microbiol. 53:1065-1073. [DOI] [PubMed] [Google Scholar]
- 32.Taylor, K. M., C. P. Kaplan, X. Gao, and A. Baker. 1996. Localization and targeting of isocitrate lyases in Saccharomyces cerevisiae. Biochem. J. 319:255-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Titorenko, V. I., J. J. Smith, R. K. Szilard, and R. A. Rachubinski. 1998. Pex20p of the yeast Yarrowia lipolytica is required for the oligomerization of thiolase in the cytosol and for its targeting to the peroxisome. J. Cell Biol. 142:403-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valenciano, S., J. R. Lucas, A. Pedregosa, I. F. Monistrol, and F. Laborda. 1996. Induction of beta-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch. Microbiol. 166:336-341. [DOI] [PubMed] [Google Scholar]
- 35.van den Bosch, H., R. B. Schutgens, R. J. Wanders, and J. M. Tager. 1992. Biochemistry of peroxisomes. Annu. Rev. Biochem. 61:157-197. [DOI] [PubMed] [Google Scholar]
- 36.Van der Leij, I., M. M. Franse, Y. Elgersma, B. Distel, and H. F. Tabak. 1993. PAS10 is a tetratricopeptide-repeat protein that is essential for the import of most matrix proteins into peroxisomes of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 90:11782-11786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Roermund, C. W., Y. Elgersma, N. Singh, R. J. Wanders, and H. F. Tabak. 1995. The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl-CoA under in vivo conditions. EMBO J. 14:3480-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Roermund, C. W., H. F. Tabak, M. van Den Bergqq, R. J. Wanders, and E. H. Hettema. 2000. Pex11p plays a primary role in medium-chain fatty acid oxidation, a process that affects peroxisome number and size in Saccharomyces cerevisiae. J. Cell Biol. 150:489-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vázquez-Torres, A., and E. Balish. 1997. Macrophages in resistance to candidiasis. Microbiol. Mol. Biol. Rev. 61:170-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vereecke, D., K. Cornelis, W. Temmerman, M. Jaziri, M. Van Montagu, M. Holsters, and K. Goethals. 2002. Chromosomal locus that affects pathogenicity of Rhodococcus fascians. J. Bacteriol. 184:1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vizeacoumar, F. J., J. C. Torres-Guzman, D. Bouard, J. D. Aitchison, and R. A. Rachubinski. 2004. Pex30p, Pex31p, and Pex32p form a family of peroxisomal integral membrane proteins regulating peroxisome size and number in Saccharomyces cerevisiae. Mol. Biol. Cell 15:665-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walther, A., and J. Wendland. 2003. An improved transformation protocol for the human fungal pathogen Candida albicans. Curr. Genet. 42:339-343. [DOI] [PubMed] [Google Scholar]
- 43.Wang, Z. Y., C. R. Thornton, M. J. Kershaw, L. Debao, and N. J. Talbot. 2003. The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea. Mol. Microbiol. 47:1601-1612. [DOI] [PubMed] [Google Scholar]
- 44.Wenzel, R. P. 1995. Nosocomial candidemia: risk factors and attributable mortality. Clin. Infect. Dis. 20:1531-1534. [DOI] [PubMed] [Google Scholar]
- 45.Wilson, R. B., D. Davis, and A. P. Mitchell. 1999. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J. Bacteriol. 181:1868-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]